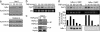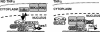Recruitment of IkappaBalpha to the hes1 promoter is associated with transcriptional repression - PubMed (original) (raw)
Recruitment of IkappaBalpha to the hes1 promoter is associated with transcriptional repression
Cristina Aguilera et al. Proc Natl Acad Sci U S A. 2004.
Abstract
The NF-kappaB pathway plays a pivotal role in proliferation, differentiation, apoptosis, and immune responses in mammals. The NF-kappaB inhibitor, IkappaB, has classically been characterized for its ability to sequester NF-kappaB transcription factors in the cytoplasm. Nevertheless, a nuclear fraction of IkappaBalpha has consistently been detected and associated with repression of nuclear NF-kappaB. Now we show that IkappaBalpha physically associates with different repression elements such as nuclear corepressors and histone acetyltransferases and deacetylases (HDACs). More remarkably, chromatin immunoprecipitation experiments demonstrate that IkappaBalpha is recruited to the promoter regions of the Notch-target gene, hes1, together with HDAC1 and -5, whereas we did not detect IkappaBalpha associated with classical NF-kappaB target genes such as IL6 and RANTES. TNF-alpha treatment results in a temporary release of IkappaBalpha from the hes1 promoter that correlates with increased histone acetylation and transcriptional activation. In addition, we demonstrate that both IkappaB kinase-alpha and -beta are simultaneously recruited to the hes1 promoter in response to TNF-alpha, coinciding with a maximum of IkappaBalpha release and gene activation. Moreover, TNF-alpha-dependent histone H3 acetylation, release of IkappaBalpha from the hes1 promoter, and hes1 mRNA synthesis are affected in IKK-alpha(-/-) mouse embryonic fibroblasts. We propose that IkappaBalpha plays a previously undescribed role in regulating the recruitment of repression elements to specific promoters. Recruitment of IKKs to the nucleus in response to TNF-alpha may induce chromatin-associated IkappaBalpha release and gene activation. These findings provide additional insight in the cross-talk between NF-kappaB and other signaling pathways.
Figures
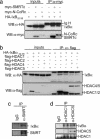
Fig. 1.
IκBα binds to multiple repressor proteins including N-CoRs and HDACs. (a) Whole-cell lysates from 293T cotransfected with the indicated plasmids were immunoprecipitated with the α-myc followed by immunoblotting with α-HA; 1/50 of the input is shown. (Lower) Immunoblotting with α-myc to detect N-CoR and SMRT. (b) Lysates from 293T cells transfected with HA-IκBα alone or cotransfected with flag-HDAC4, -5, -1, or -2 were immunoprecipitated with α-flag. Coprecipitated IκBα was detected with α-HA; 1/50 of the input is shown (Left). Western blot with the α-flag is shown as a control for precipitation. (c) 293T cell lysates were precipitated with control IgG or α-SMRTe. (Upper) Coprecipitated IκBα. (d) Endogenous HDAC1 and -5 were precipitated from 293T cells with specific antibodies. (Upper) Coprecipitated IκBα was detected with the α-IκBα by Western blot.
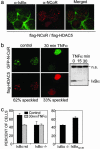
Fig. 2.
Endogenous IκBα colocalizes with N-CoR/HDAC5 nuclear speckles. (a) Colocalization of endogenous IκBα with ectopic N-CoR/HDAC-5-containing nuclear speckles [confocal image (×630)]. (b) Subcellular localization of GFP-N-CoR and flag-HDAC5 in cotransfected 293T cells before (Left) and after 30 min of TNF-α treatment (Right). A minimum of 200 cells were counted for each condition in an Olympus BX-60 microscope [confocal image (×630)]. The α-IκBα immunoblot shows TNF-α-induced IκBα degradation. (c) Percentage of cells displaying N-CoR/HDAC5 speckles in IκBα+/+ and IκBα–/– MEFs cotransfected with GFP-N-CoR and flag-HDAC5 in the control or after 30 min of TNF-α treatment (Left) or in the IκBα–/– MEFs transiently transfected with IκBα32–36 (Right). A minimum of 200 cells were counted for each condition in an Olympus BX-60 microscope.
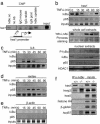
Fig. 3.
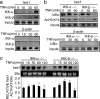
IκBα is recruited to the hes1 gene regulatory sequences. (a) Chromatin obtained from 3T3 cells was immunoprecipitated with two different α-IκBα antibodies (Santa Cruz sc-1643 and Upstate Biotechnology 06-494) or different irrelevant immunoglobulins. Recruitment of IκBα to the hes1 promoter was analyzed by PCR. (b) Effect of TNF-α treatment in the recruitment of IκBα and p65 to the hes1 promoter was analyzed by ChIP assay followed by PCR (Top). Western blot analysis showing the kinetics of total IκBα levels after TNF-α treatment and Ponceau staining as a loading control (Middle). Western blot showing the levels of nuclear IκBα or phospho-IκBα after TNF-α treatment. Nuclear levels of p65 and HDAC1 are shown as a control (Bottom). (c–e) Recruitment of IκBα and p65 to the IL6 ( c), RANTES (d), and β-actin (e) promoters was determined by ChIP assay followed by PCR analysis. All results are representative of at least two independent ChIP experiments. (f) Recruitment of IκBα to the indicated promoters analyzed by ChIP assay from IκBα+/+ and IκBα–/– MEF.
Fig. 4.
Recruitment of α-IκBα correlates with histone deacetylation and hes1 repression. (a) Chromatin prepared from TNF-α-treated 3T3 cells was immunoprecipitated with α-IκBα (Upper) and α-AcH3-K14 (Lower) antibodies. Coprecipitated DNA was analyzed by PCR with specific primers for hes1 or histone H4. Results are representative of two independent experiments. (b) TNF-α treatment induces a temporary activation of the endogenous hes1 transcription. Northern blot from 293T cells treated with TNF-α at different time points showing hes1 mRNA levels. 28s rRNA is shown as a loading control. (c) Chromatin from control or 60 min TNF-α-treated 3T3 cells was first precipitated with α-IκBα (Lower). Chromatin was then eluted and a second precipitation with α-HDAC1 or -5 antibodies was performed (Upper). The presence of hes1 promoter in the precipitates was determined by PCR analysis. (d) Northern blot showing relative hes1 transcriptional activation in IκBα+/+ (Left) and IκBα–/– (Right) MEF treated with TNF-α at the indicated time points. 28s ribosomal RNA is shown as a loading control. RNA levels were quantified, and the ratio between hes1 and 28s is represented (Lower). Induction of IκBα transcription in the IκBα+/+ is shown as a control of TNF-α activation (Lower).
Fig. 5.
IKK-α and -β are recruited to the hes1 promoter and correlate with IκBα release. (a) Chromatin prepared from TNF-α-treated 3T3 cells was immunoprecipitated with α-IKK-α or -β antibodies. Association of IKKs to the hes1 (Upper) or β-actin (Lower) genes was analyzed by PCR analysis. (b) Chromatins from TNF-α-treated IKK-α–/– and -β–/– MEFs were precipitated with α-IκBα and α-AcH3-K14 antibodies. (Upper) Recruitment of IκBα and acetylation of histone H3 on the hes1 promoter was determined by PCR analysis. (Lower) IκBα is not recruited to the β-actin gene in these cells. (c) Northern blot showing hes1 transcriptional activation in IKK-α–/– (lanes 1–5) and IKK-β–/– (lanes 6–10) MEF treated with TNF-α at the indicated time points. 28s ribosomal RNA is shown as a loading control. RNA levels were quantified and the ratio between hes1 and 28s is represented (Lower). Differences between IKK-α–/– and -β–/– were statistically significant, being the median area under the curve (AUC0min-60min) of 3.43 and 4.18, respectively (P = 0.032). One representative of three independent experiments is shown.
Fig. 6.
Model illustrates that nuclear IκBα is recruited to the hes1 promoter together with repressor elements under basal conditions. Upon TNF-α induction, IKKs are recruited to this promoter resulting in IκBα release and transcriptional activation.
Similar articles
- A nucleosomal function for IkappaB kinase-alpha in NF-kappaB-dependent gene expression.
Anest V, Hanson JL, Cogswell PC, Steinbrecher KA, Strahl BD, Baldwin AS. Anest V, et al. Nature. 2003 Jun 5;423(6940):659-63. doi: 10.1038/nature01648. Nature. 2003. PMID: 12789343 - Histone H3 phosphorylation by IKK-alpha is critical for cytokine-induced gene expression.
Yamamoto Y, Verma UN, Prajapati S, Kwak YT, Gaynor RB. Yamamoto Y, et al. Nature. 2003 Jun 5;423(6940):655-9. doi: 10.1038/nature01576. Nature. 2003. PMID: 12789342 - Histone deacetylase inhibitor trichostatin A sustains sodium pervanadate-induced NF-kappaB activation by delaying IkappaBalpha mRNA resynthesis: comparison with tumor necrosis factor alpha.
Horion J, Gloire G, El Mjiyad N, Quivy V, Vermeulen L, Vanden Berghe W, Haegeman G, Van Lint C, Piette J, Habraken Y. Horion J, et al. J Biol Chem. 2007 May 25;282(21):15383-93. doi: 10.1074/jbc.M609166200. Epub 2007 Apr 4. J Biol Chem. 2007. PMID: 17409387 - Regulation and function of IKK and IKK-related kinases.
Häcker H, Karin M. Häcker H, et al. Sci STKE. 2006 Oct 17;2006(357):re13. doi: 10.1126/stke.3572006re13. Sci STKE. 2006. PMID: 17047224 Review. - Regulation at multiple levels of NF-kappaB-mediated transactivation by protein acetylation.
Quivy V, Van Lint C. Quivy V, et al. Biochem Pharmacol. 2004 Sep 15;68(6):1221-9. doi: 10.1016/j.bcp.2004.05.039. Biochem Pharmacol. 2004. PMID: 15313420 Review.
Cited by
- Nuclear IKK activity leads to dysregulated notch-dependent gene expression in colorectal cancer.
Fernández-Majada V, Aguilera C, Villanueva A, Vilardell F, Robert-Moreno A, Aytés A, Real FX, Capella G, Mayo MW, Espinosa L, Bigas A. Fernández-Majada V, et al. Proc Natl Acad Sci U S A. 2007 Jan 2;104(1):276-81. doi: 10.1073/pnas.0606476104. Epub 2006 Dec 26. Proc Natl Acad Sci U S A. 2007. PMID: 17190815 Free PMC article. - Notch Signaling in Acute Inflammation and Sepsis.
Gallenstein N, Tichy L, Weigand MA, Schenz J. Gallenstein N, et al. Int J Mol Sci. 2023 Feb 9;24(4):3458. doi: 10.3390/ijms24043458. Int J Mol Sci. 2023. PMID: 36834869 Free PMC article. Review. - GSI-I (Z-LLNle-CHO) inhibits γ-secretase and the proteosome to trigger cell death in precursor-B acute lymphoblastic leukemia.
Meng X, Matlawska-Wasowska K, Girodon F, Mazel T, Willman CL, Atlas S, Chen IM, Harvey RC, Hunger SP, Ness SA, Winter SS, Wilson BS. Meng X, et al. Leukemia. 2011 Jul;25(7):1135-46. doi: 10.1038/leu.2011.50. Epub 2011 Apr 15. Leukemia. 2011. PMID: 21494254 Free PMC article. - NF-κB Members Left Home: NF-κB-Independent Roles in Cancer.
Colomer C, Marruecos L, Vert A, Bigas A, Espinosa L. Colomer C, et al. Biomedicines. 2017 May 25;5(2):26. doi: 10.3390/biomedicines5020026. Biomedicines. 2017. PMID: 28587092 Free PMC article. Review. - Autoactivation of the Epstein-Barr virus oncogenic protein LMP1 during type II latency through opposite roles of the NF-kappaB and JNK signaling pathways.
Goormachtigh G, Ouk TS, Mougel A, Tranchand-Bunel D, Masy E, Le Clorennec C, Feuillard J, Bornkamm GW, Auriault C, Manet E, Fafeur V, Adriaenssens E, Coll J. Goormachtigh G, et al. J Virol. 2006 Aug;80(15):7382-93. doi: 10.1128/JVI.02052-05. J Virol. 2006. PMID: 16840319 Free PMC article.
References
- Hermanson, O., Jepsen, K. & Rosenfeld, M. G. (2002) Nature 419, 934–939. - PubMed
- Jepsen, K., Hermanson, O., Onami, T. M., Gleiberman, A. S., Lunyak, V., McEvilly, R. J., Kurokawa, R., Kumar, V., Liu, F., Seto, E., et al. (2000) Cell 102, 753–763. - PubMed
- Jepsen, K. & Rosenfeld, M. G. (2002) J. Cell Sci. 115, 689–698. - PubMed
- Nagy, L., Kao, H. Y., Chakravarti, D., Lin, R. J., Hassig, C. A., Ayer, D. E., Schreiber, S. L. & Evans, R. M. (1997) Cell 89, 373–380. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous