The prototype gamma-2 herpesvirus nucleocytoplasmic shuttling protein, ORF 57, transports viral RNA through the cellular mRNA export pathway - PubMed (original) (raw)
The prototype gamma-2 herpesvirus nucleocytoplasmic shuttling protein, ORF 57, transports viral RNA through the cellular mRNA export pathway
Ben J L Williams et al. Biochem J. 2005.
Abstract
HVS (herpesvirus saimiri) is the prototype gamma-2 herpesvirus. This is a subfamily of herpesviruses gaining importance since the identification of the first human gamma-2 herpesvirus, Kaposi's sarcoma-associated herpesvirus. The HVS ORF 57 (open reading frame 57) protein is a multifunctional transregulatory protein homologous with genes identified in all classes of herpesviruses. Recent work has demonstrated that ORF 57 has the ability to bind viral RNA, shuttles between the nucleus and cytoplasm and promotes the nuclear export of viral transcripts. In the present study, we show that ORF 57 shuttles between the nucleus and cytoplasm in a CRM-1 (chromosomal region maintenance 1)-independent manner. ORF 57 interacts with the mRNA export factor REF (RNA export factor) and two other components of the exon junction complex, Y14 and Magoh. The association of ORF 57 with REF stimulates recruitment of the cellular mRNA export factor TAP (Tip-associated protein), and HVS infection triggers the relocalization of REF and TAP from the nuclear speckles to several large clumps within the cell. Using a dominant-negative form of TAP and RNA interference to deplete TAP, we show that it is essential for bulk mRNA export in mammalian cells and is required for ORF 57-mediated viral RNA export. Furthermore, we show that the disruption of TAP reduces viral replication. These results indicate that HVS utilizes ORF 57 to recruit components of the exon junction complex and subsequently TAP to promote viral RNA export through the cellular mRNA export pathway.
Figures
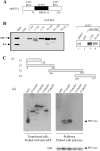
Figure 1. ORF 57 shuttles in the presence of LMB
(A) Cos-7 cells were transiently transfected with 2 μg of pRSVORF 57. After 18 h, mouse 3T3 cells were plated on to the Cos-7 cells in a medium containing cycloheximide in the absence or presence of LMB. After 4 h, the cells were washed with PBS and fused by the addition of 2 ml of 50% poly(ethylene glycol) in PBS. After washing, the cells were returned to a medium containing cycloheximide in the absence or presence of LMB for 60 min. Cells were then fixed and incubated with a 1:100 dilution of anti-ORF 57 antibody and then co-stained with 0.5 μg/ml Hoechst dye and fluorescein-conjugated anti-mouse immunoglobulin. (B) HEK-293T cells remained untransfected (lanes 1) or were transfected with pUCgB in the absence (lanes 3, 6 and 9) and presence of pRSVORF 57 (lanes 2, 4, 5, and 7, 8 and 10) or incubated in the presence of LMB (lanes 5, 8 and 11). Total (lanes 1–5), nuclear (lanes 6–8) and cytoplasmic (lanes 9–11) RNAs were then isolated and separated by elecrophoresis on a 1% denaturing formaldehyde–agarose gel. The RNA was transferred on to Hybond-N membranes and hybridized with 32P-radiolabelled random-primed probes specific for the HVS gB and 18 S rRNA-coding sequences. (C) HEK-293T cells were transiently transfected with pLUCSALRRE together with the indicated plasmids and a β-galactosidase control. Luciferase activities [RLU (relative light units)] were measured in triplicate on three separate occasions and averaged. Data were normalized for transfection efficiency by assaying for β-galactosidase activity. A schematic representation of pLUCSALRRE is shown above the graph; pCMV, CMV immediate early promoter; SD, splice donor; luc, luciferase gene; _Sal_I, unique _Sal_I restriction site; RRE, REV response element; SA, splice acceptor; pA, polyadenylation signal. (D) HEK-293T cells remained untransfected (lane 1) or were transfected with pLUCSALRRE in the absence (lane 2) and presence of pREV (lane 3) or incubated in the presence of LMB (lane 4). Total and cytoplasmic RNA was then isolated and separated by elecrophoresis on a 1% denaturing formaldehyde–agarose gel. The RNA was transferred on to Hybond-N membranes and hybridized with 32P-radiolabelled random-primed probes specific for the luciferase and 18 S rRNA-coding sequences.
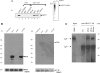
Figure 2. The N-terminal domain of ORF 57 binds to the N- and C-terminal domains of REF
(A) The domain structure for mREF2-1 is shown schematically. The central RNA recognition motif spans residues 74–153 approximately. Interactions with other proteins and RNA are shown above each domain of the protein. (B) ORF 57 binds to the N- and C-terminal domains of REF. [35S]Methionine-labelled ICP27 (right panel), ORF 57 and human Ras (left panel), synthesized in rabbit reticulocyte lysates, were incubated with beads coated with the indicated GST fusion proteins. Then, 5% of the input and 50% of the bound material were loaded on to the gel. The diffuse ORF 57 band seen in lane 4 (left panel) was caused by co-migration of ORF 57 with the GST–REF(1–153) fusion protein. (C) REF binds to the N-terminal domain of ORF 57. (i) Schematic representation of the ORF 57–GFP deletion series. (ii) Cell lysates of p57GFP, p57Δ1–4GFP and pREF-myc transfected cells were immunoprecipitated using polyclonal ORF 57 antiserum. Bound proteins were resolved by SDS/PAGE and the presence of ORF 57 was detected using anti-GFP antiserum (left panel) and REF–myc was detected using anti-myc antiserum (right panel). (iii) As a control, Western-blot analysis was performed on the supernatants before immunoprecipitation using an anti-myc antibody to confirm that REF was expressed in all samples.
Figure 3. ORF 57 (8–120) binds to REF without additional eukaryotic proteins
An aliquot of a cell lysate from E. coli expressing His6-tagged ORF 57 (8–120) is shown in lane 2 (numbering from the left), incubation of an aliquot of this lysate with Talon resin leads to the purification of ORF 57 (8–120) (lane 3). Beads coated with the indicated GST fusions were incubated with the same E. coli cell lysate containing His-tagged ORF 57 in lanes 4–7 in the presence or absence of RNase as indicated. Bound proteins were analysed by SDS/PAGE followed by Coomassie Blue staining. An aliquot of each sample was also analysed by Western blotting with an antibody specific for the His6 tag and the results are shown in (B).
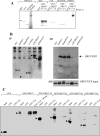
Figure 4. ORF 57 recruits TAP through REF
(A) (i) [35S]Methionine-labelled ORF 57 was synthesized in vitro and incubated with beads coated with the indicated GST or GST fusion proteins. In the control lanes, 5 μg of purified thioredoxin was added to the binding reactions, and in some binding reactions, 5 μg of purified REF was added as indicated. RNase was included in some binding reactions as indicated. The bound proteins were eluted and one-tenth of the input (lane 1) and one-third of the bound fractions (lanes 2–7) were analysed by SDS/PAGE. (ii) Radiolabelled ORF 57 mRNA generated from an in vitro transcription/translation reaction was subjected to phenol extraction of a complete GST–TAP pull-down assay in the presence or absence of RNase as indicated. The recovered RNA was analysed by denaturing PAGE and visualized using a phosphoimager. (B) HEK-293T cells were transfected with pREF-myc or pTAP-myc and then studied for three cases: uninfected, superinfected with HVS (MOI of 1) or transfected with pGFP or p57GFP. After 24 h, cell lysates were immunoprecipitated using ORF 57 antiserum. The bound proteins were resolved by SDS/PAGE and the presence of (i) REF–myc and (ii) TAP–myc were detected by Western-blot analysis. (iii) As a suitable control, Western-blot analysis was performed using REF–myc and TAP–myc on whole cell extracts to confirm that TAP and REF were expressed in all samples. (C) Western-blot analysis, using an anti-TAP Ab (antibody), of immunoprecipitates obtained with anti-ORF 57 Ab from HVS mock and infected cell extracts (24 h post-infection), after separation by SDS/PAGE: input OMK cellular extract (lane 1, numbering from the left), uninfected extract+anti-ORF 57 Ab (lane 2), infected extract+anti-ORF 57 Ab (lane 3) and infected extract+anti-ORF 57 Ab+RNase (lane 4).
Figure 5. ORF 57 associates with other components of the EJC
(A) ORF 57 interacts with components of the EJC in vitro. [35S]Methionine-labelled components of the EJC were synthesized in vitro and incubated with beads coated with the indicated GST or GST fusion proteins for REF, YI4, Mogoh and RNSP1. RNase was added where indicated. Bound proteins were eluted and one-tenth of the input (left-hand side of the gel) and one-third of the bound fractions were analysed by SDS/PAGE. (B) HEK-293T cells were transfected with myc-tagged expression constructs for REF, YI4, Magoh, RNSP1 and UPF3B (i) and in the presence of pRSVORF 57 (ii). After 24 h, cell lysates were utilized in co-immunoprecipitation analysis using an anti-myc affinity resin. Bound proteins were resolved by SDS/PAGE and the presence of ORF 57–GFP was detected using a GFP-specific antiserum. (iii) As a suitable control, Western-blot analysis was performed using anti-GFP on whole cell extracts to confirm that ORF 57–GFP was expressed in all samples. (C) Y14 associates with the N- and C-terminal domains of ORF 57. GST pull-downs were performed with GST or GST–Y14 and cell extracts from HEK-293T cells were transfected with the indicated GFP–ORF 57 fusions. RNase was added to the binding reactions where indicated. The bound proteins were detected by Western blotting using a GFP-specific antibody. The specific band corresponding to the expected size for the GFP–ORF 57 fusion is indicated in each case by an arrow.
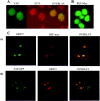
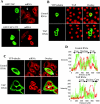
Figure 6. ORF 57 co-localizes REF and TAP in the nucleus
(A) Cos-7 cells were transfected with TAP–GFP, fixed and then immunostained with a primary antibody specific for SC35 followed by a TRITC-labelled secondary antibody. (B) HeLa cells were transfected with a myc-tagged REF expression vector and then immunostained with the anti-myc monoclonal antibody 9E10 followed by an anti-mouse FITC-labelled secondary antibody. (C) Cos-7 cells were transfected with pRSVORF 57 in the presence of (i) pREF-myc or (ii) TAP–GFP. ORF 57 was detected using an anti-ORF 57 monoclonal antiserum and (i) anti-mouse FITC-labelled secondary antibody or (ii) anti-mouse Texas Red conjugate. REF–myc was detected using a primary antibody specific for myc followed by a TRITC-labelled secondary antibody. TAP–GFP was directly visualized using fluorescence microscopy.
Figure 7. TAP is required for bulk mRNA export in mammalian cells
(A) TAP (1–372) blocks mRNA export. OMK cells were transfected with the GFP–TAP or GFP–TAP (1–372). At 48 h post-transfection, the cells were fixed, permeabilized and total mRNA was detected by FISH using a Cy3-labelled oligo(dT) probe. The arrow indicates an untransfected cell. (B) TAP can be depleted by RNAi. HeLa cells were transfected with 700 ng of pSUPERLUC (control RNAi) or 700 ng of pSUPERTAP (TAP RNAi), together with 100 ng of a YFP–tubulin expression vector. Cells were fixed and permeabilized at 96 h post-transfection. TAP was detected using a rabbit polyclonal antibody and a TRITC-labelled secondary antibody. (C) mRNA export is blocked in cells depleted for TAP. HeLa cells were transfected with 700 ng of pSUPERLUC (control RNAi) or 700 ng of pSUPERTAP (TAP RNAi) together with 100 ng of a YFP–tubulin expression vector. Cells were fixed and permeabilized 96 h post-transfection. Bulk mRNA was detected by FISH using a Cy3-labelled oligo(dT) probe. The lines through the two cells in the right panel correspond to the path for the _x_-axis of the fluorescence intensity profiles shown in (D). (D) Fluorescence intensity profiles for the control and TAP-depleted cells shown in (C); Cyto, cytoplasm. The red line corresponds to the fluorescence from the mRNA detection by FISH and the green line corresponds to the fluorescence from the YFP–tubulin.
Figure 8. Functional TAP is required for viral mRNA export
(A) HEK-293T cells remained untransfected (lane 1) or were transfected with pUCgB in the absence (lanes 4, 7 and 10) and presence of pRSVORF 57 (lanes 5, 6, 8, 9, 11 and 12) or presence of pGFP-TAP (1–372) (lanes 3, 6, 9 and 12). (B) HeLa cells remained untransfected (lane 1) or were transfected with pUCgB in the absence (lanes 4, 7 and 10) and presence of pRSVORF 57 (lanes 5, 6, 8, 9, 11 and 12) or presence of pSUPERTAP (lanes 3, 6, 9 and 12). Total (lanes 1–6), nuclear (lanes 7–9) and cytoplasmic (lanes 10–12) RNAs were then isolated and separated by electrophoresis on a 1% denaturing formaldehyde–agarose gel. The RNA was transferred on to Hybond-N membranes and hybridized with 32P-radiolabelled random-primed probes specific for the HVS gB and 18 S rRNA-coding sequences. (C) HEK-293T cells remained untransfected (lane 1) or were transfected with pGL-3 in the absence (lanes 3, 5 and 7) and presence of pRSVORF 57 (lanes 4, 6 and 8). Total (lanes 1–4), nuclear (lanes 5–6) and cytoplasmic (lanes 7–8) RNAs were then isolated and separated by electrophoresis on a 1% denaturing formaldehyde–agarose gel. The RNA was transferred on to Hybond-N membranes and hybridized with 32P-radiolabelled random-primed probes specific for the Luc and 18 S rRNA-coding sequences.
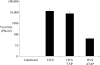
Figure 9. A dominant-negative form of TAP reduces viral replication
OMK cells remained untransfected or were transfected with pTAP-GFP or transfected with pGFP-TAP (1–372). After 24 h, the cells were infected with HVS–GFP at an MOI of 1. The supernatants were harvested 5 days post-infection and viral titres were determined using plaque assays. The variations between three replicated assays are indicated.
Similar articles
- A gamma-2 herpesvirus nucleocytoplasmic shuttle protein interacts with importin alpha 1 and alpha 5.
Goodwin DJ, Whitehouse A. Goodwin DJ, et al. J Biol Chem. 2001 Jun 8;276(23):19905-12. doi: 10.1074/jbc.M009513200. Epub 2001 Mar 16. J Biol Chem. 2001. PMID: 11278515 - The evolutionarily conserved Kaposi's sarcoma-associated herpesvirus ORF57 protein interacts with REF protein and acts as an RNA export factor.
Malik P, Blackbourn DJ, Clements JB. Malik P, et al. J Biol Chem. 2004 Jul 30;279(31):33001-11. doi: 10.1074/jbc.M313008200. Epub 2004 May 20. J Biol Chem. 2004. PMID: 15155762 - Herpesvirus saimiri ORF57: a post-transcriptional regulatory protein.
Boyne JR, Colgan KJ, Whitehouse A. Boyne JR, et al. Front Biosci. 2008 Jan 1;13:2928-38. doi: 10.2741/2898. Front Biosci. 2008. PMID: 17981766 Review. - gamma-2 Herpes virus post-transcriptional gene regulation.
Boyne JR, Whitehouse A. Boyne JR, et al. Clin Microbiol Infect. 2006 Feb;12(2):110-7. doi: 10.1111/j.1469-0691.2005.01317.x. Clin Microbiol Infect. 2006. PMID: 16441447 Review. - The open reading frame 57 gene product of herpesvirus saimiri shuttles between the nucleus and cytoplasm and is involved in viral RNA nuclear export.
Goodwin DJ, Hall KT, Stevenson AJ, Markham AF, Whitehouse A. Goodwin DJ, et al. J Virol. 1999 Dec;73(12):10519-24. doi: 10.1128/JVI.73.12.10519-10524.1999. J Virol. 1999. PMID: 10559371 Free PMC article.
Cited by
- Epstein-Barr virus protein EB2 stimulates cytoplasmic mRNA accumulation by counteracting the deleterious effects of SRp20 on viral mRNAs.
Juillard F, Bazot Q, Mure F, Tafforeau L, Macri C, Rabourdin-Combe C, Lotteau V, Manet E, Gruffat H. Juillard F, et al. Nucleic Acids Res. 2012 Aug;40(14):6834-49. doi: 10.1093/nar/gks319. Epub 2012 Apr 13. Nucleic Acids Res. 2012. PMID: 22505578 Free PMC article. - The major tegument structural protein VP22 targets areas of dispersed nucleolin and marginalized chromatin during productive herpes simplex virus 1 infection.
López MR, Schlegel EF, Wintersteller S, Blaho JA. López MR, et al. Virus Res. 2008 Sep;136(1-2):175-88. doi: 10.1016/j.virusres.2008.05.010. Epub 2008 Jun 12. Virus Res. 2008. PMID: 18584907 Free PMC article. - The role of TREX in gene expression and disease.
Heath CG, Viphakone N, Wilson SA. Heath CG, et al. Biochem J. 2016 Oct 1;473(19):2911-35. doi: 10.1042/BCJ20160010. Biochem J. 2016. PMID: 27679854 Free PMC article. Review. - Targeted disruption of Kaposi's sarcoma-associated herpesvirus ORF57 in the viral genome is detrimental for the expression of ORF59, K8alpha, and K8.1 and the production of infectious virus.
Majerciak V, Pripuzova N, McCoy JP, Gao SJ, Zheng ZM. Majerciak V, et al. J Virol. 2007 Feb;81(3):1062-71. doi: 10.1128/JVI.01558-06. Epub 2006 Nov 15. J Virol. 2007. PMID: 17108026 Free PMC article. - mRNA nuclear export and human disease.
Hurt JA, Silver PA. Hurt JA, et al. Dis Model Mech. 2008 Sep-Oct;1(2-3):103-8. doi: 10.1242/dmm.000745. Dis Model Mech. 2008. PMID: 19048072 Free PMC article.
References
- Strasser K., Masuda S., Mason P., Pfannstiel J., Oppizzi M., Rodriguez-Navarro S., Rondon A. G., Aguilera A., Struhl K., Reed R., et al. TREX is a conserved complex coupling transcription with messenger RNA export. Nature (London) 2002;417:304–308. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous