Ketamine disrupts frontal and hippocampal contribution to encoding and retrieval of episodic memory: an fMRI study - PubMed (original) (raw)
Clinical Trial
Ketamine disrupts frontal and hippocampal contribution to encoding and retrieval of episodic memory: an fMRI study
G D Honey et al. Cereb Cortex. 2005 Jun.
Abstract
The N-methyl-D-aspartate (NMDA) receptor antagonist ketamine produces episodic memory deficits. We used functional magnetic resonance imaging to characterize the effects of ketamine on frontal and hippocampal responses to memory encoding and retrieval in healthy volunteers using a double-blind, placebo-controlled, randomized, within-subjects comparison of two doses of intravenous ketamine. Dissociation of the effects of ketamine on encoding and retrieval processes was achieved using two study-test cycles: in the first, items were encoded prior to drug infusion and retrieval tested, during scanning, on drug; in the second, encoding was scanned on drug, and retrieval tested once ketamine plasma levels had declined. We additionally determined the interaction of ketamine with the depth of processing that occurred at encoding. A number of effects upon task-dependent activations were seen. Overall, our results suggest that left frontal activation is augmented by ketamine when elaborative semantic processing is required at encoding. In addition, successful encoding on ketamine is supplemented by additional non-verbal processing that is incidental to task demands. The effects of ketamine at retrieval are consistent with impaired access to accompanying contextual features of studied items. Our findings show that, even when overt behaviour is unimpaired, ketamine has an impact upon the recruitment of key regions in episodic memory task performance.
Figures
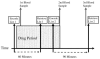
Figure 1. Diagrammatic representation of experimental protocol.
The period of drug exposure, shown in red, included the retrieval of the first study list, and encoding of the second study list.
Figure 2. Activation associated with episodic encoding during placebo treatment.
The maximum intensity projections or ‘glass brain’ figures (viewed from the right, from behind and from above, thresholded at P < 0.005, uncorrected) show activations associated with the deep versus shallow (A) and difference due to subsequent memory (Dm) (B) contrasts. Details of the activations are given in Table 3.
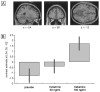
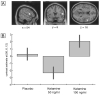
Figure 3. Dose-dependent increase in a region of left prefrontal cortex for deep compared to shallowly encoded items under ketamine compared to placebo.
Figure 4. Increased activation of right prefrontal cortex during encoding under ketamine compared to placebo, associated with items which were subsequently correctly identified, compared to incorrectly identified items.
Activation was reduced for the 50 ng/ml condition.
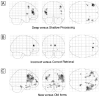
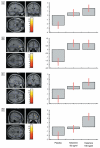
Figure 5. Activation associated with episodic retrieval during placebo treatment.
Glass brain images (threshold at P < 0.005, uncorrected). (A) Activation associated with retrieval of deep, compared to shallowly encoded items. (B) Activation associated with items which were incorrectly identified at test compared to correct responses. (C) Activation associated with items which had not been presented at encoding, compared to items previously encountered.
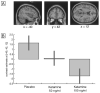
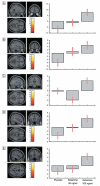
Figure 6. Dose-dependent decrease of left frontal activation for deep compared to shallowly encoded items under ketamine compared to placebo.
Figure 7. Regions demonstrating increased activation for correct compared to incorrectly identified items under ketamine compared to placebo.
Figure 8. Regions demonstrating increased activation for old compared to new items under ketamine compared to placebo.
Similar articles
- Impairment of specific episodic memory processes by sub-psychotic doses of ketamine: the effects of levels of processing at encoding and of the subsequent retrieval task.
Honey GD, Honey RA, Sharar SR, Turner DC, Pomarol-Clotet E, Kumaran D, Simons JS, Hu X, Rugg MD, Bullmore ET, Fletcher PC. Honey GD, et al. Psychopharmacology (Berl). 2005 Sep;181(3):445-57. doi: 10.1007/s00213-005-0001-z. Epub 2005 Oct 12. Psychopharmacology (Berl). 2005. PMID: 15983801 Clinical Trial. - Cognitive and subjective acute dose effects of intramuscular ketamine in healthy adults.
Lofwall MR, Griffiths RR, Mintzer MZ. Lofwall MR, et al. Exp Clin Psychopharmacol. 2006 Nov;14(4):439-49. doi: 10.1037/1064-1297.14.4.439. Exp Clin Psychopharmacol. 2006. PMID: 17115871 Clinical Trial. - Depth of processing effects on neural correlates of memory encoding: relationship between findings from across- and within-task comparisons.
Otten LJ, Henson RN, Rugg MD. Otten LJ, et al. Brain. 2001 Feb;124(Pt 2):399-412. doi: 10.1093/brain/124.2.399. Brain. 2001. PMID: 11157567 Clinical Trial. - Encoding and immediate retrieval tasks in patients with epilepsy: A functional MRI study of verbal and visual memory.
Saddiki N, Hennion S, Viard R, Ramdane N, Lopes R, Baroncini M, Szurhaj W, Reyns N, Pruvo JP, Delmaire C. Saddiki N, et al. J Neuroradiol. 2018 May;45(3):157-163. doi: 10.1016/j.neurad.2018.02.003. Epub 2018 Mar 1. J Neuroradiol. 2018. PMID: 29501535 Review. - Acute and chronic effects of ketamine upon human memory: a review.
Morgan CJ, Curran HV. Morgan CJ, et al. Psychopharmacology (Berl). 2006 Nov;188(4):408-24. doi: 10.1007/s00213-006-0572-3. Epub 2006 Sep 28. Psychopharmacology (Berl). 2006. PMID: 17006715 Review.
Cited by
- Effects of ketamine on brain function during metacognition of episodic memory.
Lehmann M, Neumann C, Wasserthal S, Schultz J, Delis A, Trautner P, Hurlemann R, Ettinger U. Lehmann M, et al. Neurosci Conscious. 2021 Feb 10;2021(1):niaa028. doi: 10.1093/nc/niaa028. eCollection 2021. Neurosci Conscious. 2021. PMID: 33747545 Free PMC article. - Ketamine pharmacology: an update (pharmacodynamics and molecular aspects, recent findings).
Mion G, Villevieille T. Mion G, et al. CNS Neurosci Ther. 2013 Jun;19(6):370-80. doi: 10.1111/cns.12099. Epub 2013 Apr 10. CNS Neurosci Ther. 2013. PMID: 23575437 Free PMC article. - Effects of early ketamine exposure on cerebral gray matter volume and functional connectivity.
Hung CC, Liu YH, Huang CC, Chou CY, Chen CM, Duann JR, Li CR, Lee TS, Lin CP. Hung CC, et al. Sci Rep. 2020 Sep 23;10(1):15488. doi: 10.1038/s41598-020-72320-z. Sci Rep. 2020. PMID: 32968108 Free PMC article. - High-dose glycine treatment of refractory obsessive-compulsive disorder and body dysmorphic disorder in a 5-year period.
Cleveland WL, DeLaPaz RL, Fawwaz RA, Challop RS. Cleveland WL, et al. Neural Plast. 2009;2009:768398. doi: 10.1155/2009/768398. Epub 2010 Feb 18. Neural Plast. 2009. PMID: 20182547 Free PMC article. - Gene expression changes in GABA(A) receptors and cognition following chronic ketamine administration in mice.
Tan S, Rudd JA, Yew DT. Tan S, et al. PLoS One. 2011;6(6):e21328. doi: 10.1371/journal.pone.0021328. Epub 2011 Jun 21. PLoS One. 2011. PMID: 21712993 Free PMC article.
References
- Adler CM, Goldberg TE, Malhotra AK, Pickar D, Breier A. Effects of ketamine on thought disorder, working memory, and semantic memory in healthy volunteers. Biol Psychiatry. 1998;43:811–816. - PubMed
- Adler CM, Malhotra AK, Elman I, Goldberg T, Egan M, Pickar D, Breier A. Comparison of ketamine-induced thought disorder in healthy volunteers and thought disorder in schizophrenia. Am J Psychiatry. 1999;156:1646–1649. - PubMed
- Brebion G, Smith MJ, Widlocher D. Discrimination and response bias in memory: effects of depression severity and psychomotor retardation. Psychiatry Res. 1997;70:95–103. - PubMed
- Breier A, Malhotra AK, Pinals DA, Weisenfeld NI, Pickar D. Association of ketamine-induced psychosis with focal activation of the prefrontal cortex in healthy volunteers. Am J Psychiatry. 1997;154:805–811. - PubMed
- Brewer JB, Zhao Z, Desmond JE, Glover GH, Gabrieli JD. Making memories: brain activity that predicts how well visual experience will be remembered. Science. 1998;281:1185–1187. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical