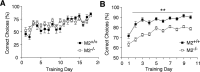M2 muscarinic acetylcholine receptor knock-out mice show deficits in behavioral flexibility, working memory, and hippocampal plasticity - PubMed (original) (raw)
M2 muscarinic acetylcholine receptor knock-out mice show deficits in behavioral flexibility, working memory, and hippocampal plasticity
Thomas Seeger et al. J Neurosci. 2004.
Abstract
Muscarinic acetylcholine receptors are known to play key roles in facilitating cognitive processes. However, the specific roles of the individual muscarinic receptor subtypes (M1-M5) in learning and memory are not well understood at present. In the present study, we used wild-type (M2+/+) and M2 receptor-deficient (M2-/-) mice to examine the potential role of M2 receptors in learning and memory and hippocampal synaptic plasticity. M2-/- mice showed significant deficits in behavioral flexibility and working memory in the Barnes circular maze and the T-maze delayed alternation tests, respectively. The behavioral deficits of M2-/- mice were associated with profound changes in neuronal plasticity studied at the Schaffer-CA1 synapse of hippocampal slices. Strikingly, short-term potentiation (STP) was abolished, and long-term potentiation (LTP) was drastically reduced after high-frequency stimulation of M2-/- hippocampi. Treatment of M2-/- hippocampal slices with the GABA(A) receptor antagonist, bicuculline, restored STP and significantly increased LTP. Whole-cell recordings from CA1 pyramidal cells demonstrated a much stronger disinhibition of GABAergic than glutamatergic transmission in M2-/- hippocampi, which was particularly prominent during stimulus trains. Increased strength of GABAergic inhibition is thus a likely mechanism underlying the impaired synaptic plasticity observed with M2-/- hippocampi. Moreover, the persistent enhancement of excitatory synaptic transmission in CA1 pyramidal cells induced by the transient application of a low concentration of a muscarinic agonist (referred to as LTP(m)) was totally abolished in M2-/- mice. Because impaired muscarinic cholinergic neurotransmission is associated with Alzheimer's disease and normal aging processes, these findings should be of considerable therapeutic relevance.
Figures
Figure 1.
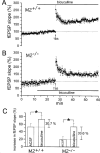
M2-/- mice show performance deficits during the first week of testing in the Barnes circular maze. A, Escape latencies. During training days 1-5, M2-/- mice took significantly more time than M2+/+ mice to locate the escape tunnel (significantly different from M2+/+ mice, *p < 0.05; two-way ANOVA, Bonferroni's post hoc comparison matrix). The escape latencies significantly decreased across training days in both M2-/- and M2+/+ mice (p < 0.01; two-way ANOVA). B, Error rates. During days 1-5, M2-/- mice made significantly more errors (defined as visits to any nontarget hole) than M2+/+ mice (significantly different from M2+/+ mice, **p < 0.01; two-way ANOVA, Bonferroni's post hoc comparison matrix). The number of errors decreased across training days in both M2-/- and M2+/+ mice (p < 0.01; two-way ANOVA). Values are given as means ± SEM (M2+/+, n = 9; M2-/-, n = 11).
Figure 2.
M2-/- mice are impaired in reversal learning in the Barnes circular maze. A, Escape latencies during reversal learning in the Barnes circular maze test. For reversal learning experiments, the escape tunnel was moved to a new position (opposite of the original; for details, see Materials and Methods). Mice were then examined for their ability to locate the new position of the escape hole. M2-/- mice showed significantly longer escape latencies than M2+/+ mice (**p < 0.005; t test). B, Number (No.) of perseverations. M2-/- mice showed significantly more perseverations (defined as repeated visits to the original hole or one of the two adjacent holes) than M2+/+ mice during reversal learning (**p < 0.005; t test). Values represent the means ± SEM of five consecutive trials (M2+/+, n = 9; M2-/-, n = 11).
Figure 3.
M2-/- mice show deficits in a T-maze test (spatial delayed alternation). T-maze experiments (spatial delayed alternation task) were performed as described in Materials and Methods, either with a 5 sec (A) or 20 sec (B) intertrial interval. In sessions with a 5 sec delay, there was no significant difference in the percentage of correct arm choices made by M2+/+ and M2-/- mice. In contrast, in sessions with a 20 sec delay, M2-/- mice made significantly fewer correct choices than M2+/+ mice (significantly different from M2+/+ mice, **p < 0.01; two-way ANOVA, Bonferroni's post hoc comparison matrix). Values represent the percentage of correct choices per session (maximum possible number of correct choices, 10). Data are given as means ± SEM (M2+/+, n = 14; M2-/-, n = 15).
Figure 4.
Carbachol-induced LTP (LTPm) is absent in hippocampal slices from M2-/- mice. Superfusion of M2+/+ hippocampal slices (filled squares; n = 9) with a low concentration (0.5 μ
m
) of CCh for 20 min induced a pronounced enhancement of fEPSPs (LTPm; filled squares; n = 9). In contrast, the same protocol failed to evoke LTPm in M2-/- hippocampi (open squares; n = 9).
Figure 5.
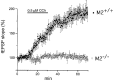
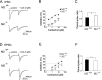
STP is absent, and LTP is greatly reduced in hippocampal slices from M2-/- mice. A, fEPSPs were recorded in CA1 stratum radiatum of M2+/+ and M2-/- hippocampi before TBS (trace 1), in the initial phase of STP (trace 2), and after induction of LTP (trace 3). The illustrated recordings were taken at the like-numbered time points indicated in B. Each trace is an average of four sweeps. B, A comparison of TBS-induced changes in the slope of fEPSPs between M2+/+ (filled squares) and M2-/- (open squares) hippocampi demonstrates the lack of STP and impaired induction of LTP in M2-/- mice. fEPSPs slopes were normalized to 100% before TBS. Under control conditions, fEPSP slopes did not differ between M2+/+ (0.194 ± 0.033 mV/msec; n = 9) and M2-/- (0.188 ± 0.022 mV/msec; n = 8; p > 0.7) hippocampal preparations.
Figure 6.
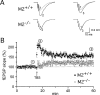
In M2+/+ hippocampal slices, the M2 receptor-preferring antagonist gallamine abrogates STP and impairs LTP but enhances control fEPSPs. A, In M2+/+ hippocampal preparations, application of gallamine (20 μ
m
) alone enhanced fEPSPs by ∼25%. This effect was abolished in M2-/- hippocampi (B; n = 5), suggesting that low-frequency synaptic excitation of CA1 pyramidal cells is tonically inhibited by ambient acetylcholine acting on M2 receptors. As observed with M2-/- hippocampal preparations, TBS failed to induce STP and robust LTP in gallamine-treated M2+/+ hippocampi (n = 12). Both parameters of synaptic plasticity were reliably evoked after wash-out of gallamine.
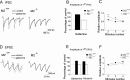
Figure 7.
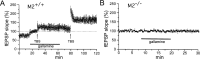
The GABAA receptor antagonist bicuculline restores STP and improves LTP in hippocampal slices from M2-/- mice. A, B, Effect of bicuculline (10 μ
m
) in M2+/+ (A) and M2-/- (B) hippocampi. GABAA receptor blockade restores STP in M2-/- hippocampi. C compares LTP (determined 30 min after TBS and expressed as percentage increase in fEPSP slopes relative to baseline) in M2+/+ and M2-/- hippocampal preparations in the absence (ACSF) or presence of bicuculline. Note that the LTP-promoting action of bicuculline is more pronounced in M2-/- than in M2+/+ hippocampi. *p < 0.01 (M2+/+, n = 5; M2-/-, n = 6).
Figure 8.
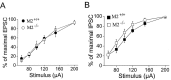
Input-output relationships for EPSCs (A) and IPSCs (B) in CA1 pyramidal cells of M2+/+ and M2-/- hippocampi. EPSCs (M2+/+, n = 8; M2-/-, n = 7) and IPSCs (M2+/+, n = 8; M2-/-, n = 8) were normalized to their maximal amplitude. The moderate leftward shift of the I-O curve for IPSCs in M2-/- hippocampi did not reach statistical significance.
Figure 9.
Effects of CCh, a nonselective cholinergic agonist, on IPSCs and EPSCs of M2+/+ and M2-/- hippocampi. A, D, Synaptic current responses were evoked using a paired-pulse protocol in which IPSCs displayed paired-pulse depression, whereas EPSCs displayed paired-pulse facilitation. B, E, CCh dose dependently reduced IPSCs (n = 11) and EPSCs (n = 9) in M2+/+ hippocampi. Note the higher potency of CCh at inhibitory versus excitatory synapses of M2+/+ hippocampi. In M2-/- hippocampi, the efficacy of CCh to suppress synaptic responses was much more attenuated for IPSCs (n = 10) than for EPSCs (n = 8). Dose-response curves depict the relative inhibition of the first synaptic response during paired-pulse stimulation. Each data point is the average of five to nine measurements. C, F, The strong paired-pulse depression of IPSCs found in M2+/+ mice (ratio 0.59 ± 0.04; n = 11) was significantly alleviated in M2-/- hippocampi (ratio, 0.72 ± 0.03; n = 10), whereas paired-pulse facilitation of EPSCs did not vary between M2+/+ (ratio, 1.53 ± 0.08; n = 9) and M2-/- hippocampi (ratio, 1.43 ± 0.05; n = 8). *p < 0.05; **p < 0.01. NS, Not significantly different.
Figure 10.
Response pattern of IPSCs and EPSCs during repetitive stimulation in M2+/+ and M2-/- hippocampi. A, The strong depression of IPSCs in M2+/+ hippocampi (left; n = 15) was substantially attenuated by the M2 receptor-preferring antagonist gallamine (20 μ
m
; n = 8). In M2-/- hippocampi, the IPSC response displayed much less depression (right; n = 11), and gallamine was rendered ineffective (n = 6). B, The histogram summarizes the experiments shown in A and compares the effect of gallamine on the amplitudes of the first IPSC in a stimulus train. C, To compare quantitatively the degree of depression between M2+/+ (n = 15) and M2-/- hippocampi (n = 11), the amplitudes of the second to fourth IPSCs were normalized to that of the first IPSC. D, Facilitation of EPSCs in M2+/+ (left side) and M2-/- hippocampi (right side) in the absence (M2+/+, n = 10; M2-/-, n = 10) and presence of 20 μ
m
gallamine (M2+/+, n = 7; M2-/-, n = 8). E, The histogram summarizes the effects of 20 μ
m
gallamine and 1-2 μ
m
atropine (n = 4) on the amplitude of the first EPSC in a stimulus train. F, Quantitative comparison of EPSC facilitation between M2+/+ (n = 10) and M2-/- hippocampi (n = 10) showed a trend toward less facilitation in M2-/- preparations, which, however, did not reach statistical significance. *p < 0.05.
Similar articles
- M2 muscarinic acetylcholine receptors regulate long-term potentiation at hippocampal CA3 pyramidal cell synapses in an input-specific fashion.
Zheng F, Wess J, Alzheimer C. Zheng F, et al. J Neurophysiol. 2012 Jul;108(1):91-100. doi: 10.1152/jn.00740.2011. Epub 2012 Apr 4. J Neurophysiol. 2012. PMID: 22490561 Free PMC article. - Activation of Muscarinic M1 Acetylcholine Receptors Induces Long-Term Potentiation in the Hippocampus.
Dennis SH, Pasqui F, Colvin EM, Sanger H, Mogg AJ, Felder CC, Broad LM, Fitzjohn SM, Isaac JT, Mellor JR. Dennis SH, et al. Cereb Cortex. 2016 Jan;26(1):414-26. doi: 10.1093/cercor/bhv227. Epub 2015 Oct 15. Cereb Cortex. 2016. PMID: 26472558 Free PMC article. - Shedding light on cholecystokinin's role in hippocampal neuroplasticity and memory formation.
Asim M, Wang H, Chen X. Asim M, et al. Neurosci Biobehav Rev. 2024 Apr;159:105615. doi: 10.1016/j.neubiorev.2024.105615. Epub 2024 Mar 2. Neurosci Biobehav Rev. 2024. PMID: 38437975 Review. - Assessment of spatial memory in mice.
Sharma S, Rakoczy S, Brown-Borg H. Sharma S, et al. Life Sci. 2010 Oct 23;87(17-18):521-36. doi: 10.1016/j.lfs.2010.09.004. Epub 2010 Sep 15. Life Sci. 2010. PMID: 20837032 Free PMC article. Review.
Cited by
- Molecular mechanisms underlying the neural correlates of working memory.
Xu X, Zhao H, Song Y, Cai H, Zhao W, Tang J, Zhu J, Yu Y. Xu X, et al. BMC Biol. 2024 Oct 21;22(1):238. doi: 10.1186/s12915-024-02039-0. BMC Biol. 2024. PMID: 39428484 Free PMC article. - Muscarinic Receptors and Alzheimer's Disease: New Perspectives and Mechanisms.
Monaco M, Trebesova H, Grilli M. Monaco M, et al. Curr Issues Mol Biol. 2024 Jul 2;46(7):6820-6835. doi: 10.3390/cimb46070407. Curr Issues Mol Biol. 2024. PMID: 39057049 Free PMC article. Review. - M2 receptors are required for spatiotemporal sequence learning in mouse primary visual cortex.
Sarkar S, Martinez Reyes C, Jensen CM, Gavornik JP. Sarkar S, et al. J Neurophysiol. 2024 Jun 1;131(6):1213-1225. doi: 10.1152/jn.00016.2024. Epub 2024 Apr 17. J Neurophysiol. 2024. PMID: 38629848 - Interaction of cholinergic disruption and age on cognitive flexibility in rats.
Cammarata C, De Rosa ED. Cammarata C, et al. Exp Brain Res. 2022 Nov;240(11):2989-2997. doi: 10.1007/s00221-022-06472-x. Epub 2022 Oct 5. Exp Brain Res. 2022. PMID: 36198843 Free PMC article.
References
- Anagnostaras SG, Maren S, Fanselow MS (1995) Scopolamine selectively disrupts the acquisition of contextual fear conditioning in rats. Neurobiol Learn Mem 64: 191-194. - PubMed
- Anagnostaras SG, Murphy GG, Hamilton SE, Mitchell SL, Rahnama NP, Nathanson NM, Silva AJ (2003) Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat Neurosci 6: 51-58. - PubMed
- Auerbach JM, Segal M (1994) A novel cholinergic induction of long-term potentiation in rat hippocampus. J Neurophysiol 72: 2034-2040. - PubMed
- Bach ME, Hawkins RD, Osman M, Kandel ER, Mayford M (1995) Impairment of spatial but not contextual memory in CaMKII mutant mice with a selective loss of hippocampal LTP in the range of the theta frequency. Cell 81: 905-915. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous