Defined types of cortical interneurone structure space and spike timing in the hippocampus - PubMed (original) (raw)
Review
Defined types of cortical interneurone structure space and spike timing in the hippocampus
Peter Somogyi et al. J Physiol. 2005.
Abstract
The cerebral cortex encodes, stores and combines information about the internal and external environment in rhythmic activity of multiple frequency ranges. Neurones of the cortex can be defined, recognized and compared on the comprehensive application of the following measures: (i) brain area- and cell domain-specific distribution of input and output synapses, (ii) expression of molecules involved in cell signalling, (iii) membrane and synaptic properties reflecting the expression of membrane proteins, (iv) temporal structure of firing in vivo, resulting from (i)-(iii). Spatial and temporal measures of neurones in the network reflect an indivisible unity of evolutionary design, i.e. neurones do not have separate structure or function. The blueprint of this design is most easily accessible in the CA1 area of the hippocampus, where a relatively uniform population of pyramidal cells and their inputs follow an instantly recognizable laminated pattern and act within stereotyped network activity patterns. Reviewing the cell types and their spatio-temporal interactions, we suggest that CA1 pyramidal cells are supported by at least 16 distinct types of GABAergic neurone. During a given behaviour-contingent network oscillation, interneurones of a given type exhibit similar firing patterns. During different network oscillations representing two distinct brain states, interneurones of the same class show different firing patterns modulating their postsynaptic target-domain in a brain-state-dependent manner. These results suggest roles for specific interneurone types in structuring the activity of pyramidal cells via their respective target domains, and accurately timing and synchronizing pyramidal cell discharge, rather than providing generalized inhibition. Finally, interneurones belonging to different classes may fire preferentially at distinct time points during a given oscillation. As different interneurones innervate distinct domains of the pyramidal cells, the different compartments will receive GABAergic input differentiated in time. Such a dynamic, spatio-temporal, GABAergic control, which evolves distinct patterns during different brain states, is ideally suited to regulating the input integration of individual pyramidal cells contributing to the formation of cell assemblies and representations in the hippocampus and, probably, throughout the cerebral cortex.
Figures
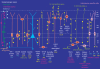
Figure 1. Innervation of pyramidal cells by 12 types of GABAergic interneuron and interneurons by 4 types of interneuron specific cell in the CA1 area of the hippocampus
The main lamina specific glutamatergic inputs are indicated on the left. The somata and dendrites of interneurons innervating pyramidal cells are shown in orange, those innervating mainly or exclusively other interneurons are shown in pink. Axons are shown in light green and the main termination zone of GABAergic synapses are shown by yellow symbols. The proposed names of neurons, some of them abbreviated, are under each schematic cell and a minimal list of molecular cell markers is given, which in combination with the axonal patterns help the recognition and characterisation of each class. Note that one molecular cell marker may be expressed by several distinct cell types. Some cells are listed on the basis of limited data from one study and further data may lead to lumping of some classes (see text). Some additional cell types, which have not been reported in sufficient detail, are not indicated. Note the association of the output synapses of different sets of cell types with the perisomatic region, and either the Schaffer collateral, commissural or the entorhinal pathway termination zones, respectively. CB, calbindin; CR, calretinin; LM-PP, lacunosum-moleculare–perforant path; LM-R-PP; lacunosum-moleculare–radiatum–perforant path; m2, muscarinic receptor type 2; NPY, neuropeptide tyrosine; PV, parvalbumin; SM, somatostatin; VGLUT3, vesicular glutamate transporter 3.
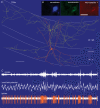
Figure 2. Basket cells phase and synchronize pyramidal cell firing in the CA1 area_in vitro_
A, paired intracellular recording of an electron microscopically defined basket cell (orange traces) and a pyramidal cell (blue traces). When the pyramidal cell was depolarized to fire at low rate, the action potentials following the basket cell-evoked IPSP (onset, orange triangle) fell in a narrow window (top trace), due to the resetting of an intrinsic membrane potential oscillation (superimposition of 12 sweeps). This is apparent in the average of those sweeps in which the pyramidal cell did not fire (middle trace), showing a depolarizing overshoot at the time interval when the pyramidal cell produces rebound spikes. Hyperpolarizing the pyramidal cell by constant current injection eliminates the oscillation (bottom trace). B, a single IPSP of unitary amplitude, evoked by extracellular stimulation close to the pyramidal cell layer (onset, orange triangle), synchronized the firing of two tonically discharging intracellularly recorded pyramidal cells (PC1, PC2) at theta frequency for several cycles due to the resetting of intrinsic membrane oscillations (traces superimposed on the onset of the IPSP). C, when the IPSPs were evoked at theta frequency (triangles), the synchrony became much tighter due to the sequential inhibition and facilitation of firing probability in narrow time windows. Scales in A: A, B refers to two top panels; C, D refers to bottom two panels. Data from Cobb et al. (1995) with permission (
); panel A was prepared by the late Eberhard Buhl.
Figure 3. In vivo firing patterns and visualization of a bistratified cell (T83a)
A, reconstruction of the neurobiotin-labelled bistratified cell. The soma and dendrites (orange) are shown complete; the axon (yellow) is shown only from 3 sections of 65 µm thickness for clarity. Note that the axon branches preferentially in stratum radiatum (str. rad.) and stratum oriens (str. or.), but avoids stratum pyramidale (str. pyr.) and stratum lacunosum-moleculare. Reconstruction made by and presented courtesy of Peter Szucs. Scale bar, 100 µm. B, immunofluorescence micrographs showing that the bistratified cell expressed the neuropeptides somatostatin and neuropeptide Y. Scale bar 20 µm. C, in vivo firing patterns of the cell showing the ripples (local field potential filtered 90–140 Hz), local field potential in the pyramidal cell layer (filtered 0.3–200 Hz) and spikes of the labelled cell (filtered 0.8–5 kHz). Note that during the initial theta oscillations (4 Hz) the cell fired rhythmically on the trough of the theta cycles. Subsequently, the local field potential became more irregular and sharp wave-associated ripples appeared, around which the cell strongly increased firing. Scales: 0.5 s; ripples 0.1 mV; local field potential 1 mV; spikes 0.2 mV.
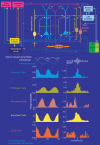
Figure 4. In vivo firing patterns of four types of interneurone during two oscillatory network states
Action potentials (filtered 0.8–5 kHz) of a parvalbumin immunopositive basket, an axo-axonic, a bistratified and an O-LM cell are shown during theta oscillations (3–6 Hz) and ripple episodes (filtered 90–140 Hz) recorded extracellularly in the pyramidal cell layer. During theta oscillations the PV basket cell fired at the descending phase, the axo-axonic cell around the peak and the bistratified and the O-LM cell discharged mainly at the trough of theta wave. Note the different inter-spike intervals during single theta cycles between bistratified and O-LM cells. During ripple episodes the PV basket cell fired preferentially at the highest amplitude of the ripple, the axo-axomic cell sometimes fired before the ripple but was depressed afterwards, the bistratified cell fired throughout, before and after the ripple episode and the O-LM cell was silent. Scales: ripples, 0.1 mV, 50 ms; spikes, 0.5 mV; theta, 0.2 mV, 0.3 s. (Data modified from Klausberger et al. 2003, .)
Figure 5. Synaptic connectivity and distinct in vivo firing patterns of pyramidal cells and four types of interneurone embedded in the hippocampal network
The schematic drawing summarizes the main synaptic connections in the CA1 area of pyramidal cells (blue), parvalbumin expressing basket, axo-axonic, bistratified and O-LM cells. The cells have differential temporal firing patterns during theta and ripple oscillations (mean of several cells). For clarity two theta cycles are shown in the firing probability histograms. The Y axis of the spike probability plots was constructed by including all events and cycles in the analysed period irrespective of whether the individual recorded cell fired or not. The phase relationship of the extracellularly recorded field potential (schematic white wave) used in the spike alignments and the phase shifted oscillation in the membrane potential oscillation of pyramidal cells reported from intracellular studies (blue waves) is shown schematically. For the ripples, time was normalized to the beginning, highest amplitude and end of ripple episode. The spike probability plots show that during different network oscillations representing two distinct brain states, interneurones of the same connectivity class show different firing activities and therefore modulate their specific postsynaptic target-domain in a brain-state-dependent manner. Interneurones belonging to different connectivity classes fire preferentially at distinct time points during a given oscillation. Because the different interneurones innervate distinct domains of the pyramidal cells, the respective compartments will receive GABAergic input at different time points. This suggests a role for interneurones in the temporal structuring of the activity of pyramidal cells and their inputs via their respective target domain in a co-operative manner, rather than simply providing generalized inhibition. (Firing probability histograms modified from Klausberger et al. 2003, .)
Similar articles
- Intrinsic and synaptic mechanisms determining the timing of neuron population activity during hippocampal theta oscillation.
Orbán G, Kiss T, Erdi P. Orbán G, et al. J Neurophysiol. 2006 Dec;96(6):2889-904. doi: 10.1152/jn.01233.2005. Epub 2006 Aug 9. J Neurophysiol. 2006. PMID: 16899632 - GABAergic contributions to gating, timing, and phase precession of hippocampal neuronal activity during theta oscillations.
Cutsuridis V, Hasselmo M. Cutsuridis V, et al. Hippocampus. 2012 Jul;22(7):1597-621. doi: 10.1002/hipo.21002. Epub 2012 Jan 18. Hippocampus. 2012. PMID: 22252986 - Hippocampal gamma-frequency oscillations: from interneurones to pyramidal cells, and back.
Mann EO, Radcliffe CA, Paulsen O. Mann EO, et al. J Physiol. 2005 Jan 1;562(Pt 1):55-63. doi: 10.1113/jphysiol.2004.078758. Epub 2004 Nov 11. J Physiol. 2005. PMID: 15539391 Free PMC article. Review. - Input and frequency-specific entrainment of postsynaptic firing by IPSPs of perisomatic or dendritic origin.
Tamás G, Szabadics J, Lörincz A, Somogyi P. Tamás G, et al. Eur J Neurosci. 2004 Nov;20(10):2681-90. doi: 10.1111/j.1460-9568.2004.03719.x. Eur J Neurosci. 2004. PMID: 15548211
Cited by
- Cl⁻ homeodynamics in gap junction-coupled astrocytic networks on activation of GABAergic synapses.
Egawa K, Yamada J, Furukawa T, Yanagawa Y, Fukuda A. Egawa K, et al. J Physiol. 2013 Aug 15;591(16):3901-17. doi: 10.1113/jphysiol.2013.257162. Epub 2013 Jun 3. J Physiol. 2013. PMID: 23732644 Free PMC article. - On initial Brain Activity Mapping of episodic and semantic memory code in the hippocampus.
Tsien JZ, Li M, Osan R, Chen G, Lin L, Wang PL, Frey S, Frey J, Zhu D, Liu T, Zhao F, Kuang H. Tsien JZ, et al. Neurobiol Learn Mem. 2013 Oct;105:200-10. doi: 10.1016/j.nlm.2013.06.019. Epub 2013 Jul 6. Neurobiol Learn Mem. 2013. PMID: 23838072 Free PMC article. Review. - NECAB1-3, parvalbumin, calbindin, and calretinin in the hippocampus of the European mole.
Maliković J, Amrein I, Vinciguerra L, Wolfer DP, Slomianka L. Maliković J, et al. Front Neuroanat. 2024 Sep 4;18:1452722. doi: 10.3389/fnana.2024.1452722. eCollection 2024. Front Neuroanat. 2024. PMID: 39296922 Free PMC article. - Cognitive Deficits in Calsyntenin-2-deficient Mice Associated with Reduced GABAergic Transmission.
Lipina TV, Prasad T, Yokomaku D, Luo L, Connor SA, Kawabe H, Wang YT, Brose N, Roder JC, Craig AM. Lipina TV, et al. Neuropsychopharmacology. 2016 Feb;41(3):802-10. doi: 10.1038/npp.2015.206. Epub 2015 Jul 14. Neuropsychopharmacology. 2016. PMID: 26171716 Free PMC article. - A Mouse Model of Neurodegeneration Induced by Blade Penetrating Stab Wound to the Hippocampus.
He BD, Liu CM, Teng ZQ. He BD, et al. Biology (Basel). 2022 Sep 18;11(9):1365. doi: 10.3390/biology11091365. Biology (Basel). 2022. PMID: 36138848 Free PMC article.
References
- Acsady L, Arabadzisz D, Freund TF. Correlated morphological and neurochemical features identify different subsets of vasoactive intestinal polypeptide-immunoreactive interneurons in rat hippocampus. Neuroscience. 1996a;73:299–315. - PubMed
- Acsady L, Gorcs TJ, Freund TF. Different populations of vasoactive intestinal polypeptide-immunoreactive interneurons are specialized to control pyramidal cells or interneurons in the hippocampus. Neuroscience. 1996b;73:317–334. - PubMed
- Ali AB, Bannister AP, Thomson AM. IPSPs elicited in CA1 pyramidal cells by putative basket cells in slices of adult rat hippocampus. Eur J Neurosci. 1999;11:1741–1753. - PubMed
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–591. - PubMed
- Andersen P, Eccles JC, Loyning Y. Recurrent inhibition in the hippocampus with identification of the inhibitory cell and its synapses. Nature. 1963;198:540–542. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous