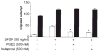Prostaglandin E(2) inhibits fibroblast migration by E-prostanoid 2 receptor-mediated increase in PTEN activity - PubMed (original) (raw)
Prostaglandin E(2) inhibits fibroblast migration by E-prostanoid 2 receptor-mediated increase in PTEN activity
Eric S White et al. Am J Respir Cell Mol Biol. 2005 Feb.
Erratum in
- Am J Respir Cell Mol Biol. 2008 Aug;39(2):252
Abstract
An increased migratory phenotype exists in lung fibroblasts derived from patients with fibroproliferative lung disease. Prostaglandin E(2) (PGE(2)) suppresses fibroblast migration, but the receptor(s) and mechanism(s) mediating this action are unknown. Our data confirm that treatment of human lung fibroblasts with PGE(2) inhibits migration. Similar effects of butaprost, an E-prostanoid (EP) 2 receptor-specific ligand, implicate the EP2 receptor in migration-inhibitory signaling. Further, migration in fibroblasts deficient for the EP2 receptor cannot be inhibited by PGE(2) or butaprost, confirming the central role of EP2 in mediating these effects. Our previous data suggested that phosphatase and tensin homolog on chromosome ten (PTEN), a phosphatase that opposes the actions of phosphatidylinositol-3-kinase (PI3K), may be important in regulating lung fibroblast motility. We now report that both PGE(2) and butaprost increase PTEN phosphatase activity, without a concomitant increase in PTEN protein levels. This contributes to EP2-mediated migration inhibition, because migration in PTEN-null fibroblasts is similarly unaffected by EP2 receptor signaling. Increased PTEN activity in response to EP2 stimulation is associated with decreased tyrosine phosphorylation on PTEN, a mechanism known to regulate enzyme activity. Collectively, these data describe the novel mechanistic finding that PGE(2), via the EP2 receptor, decreases tyrosine phosphorylation on PTEN, resulting in increased PTEN enzyme activity and inhibition of fibroblast migration.
Conflict of interest statement
Conflict of Interest Statement : E.S.W. has no declared conflicts of interest; R.G.A. has no declared conflicts of interest; E.G.D. has no declared conflicts of interest; D.M.A. has no declared conflicts of interest; V.S. has no declared conflicts of interest; T.W.M. has no declared conflicts of interest; B.B.M. has no declared conflicts of interest; and M.P-G. has no declared conflicts of interest.
Figures
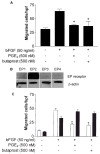
Figure 1
PGE2 inhibits bFGF-induced fibroblast migration. (A ) Migration of human lung fibroblasts in the presence or absence of bFGF, PGE2, or butaprost. Results are expressed as the mean number of migrated cells per hpf ± SEM. Experiments were performed in triplicate wells, and the experiment was repeated twice with representative results shown (*P < 0.001). (B ) The EP2 receptor is the predominant EP receptor expressed on IMR-90 cells. Bands corresponding to the EP1 and EP3 receptors were present but extremely faint. EP4 was undetectable. The results are representative of two separate experiments. (C ) Migration of wild-type (C57Bl/6) (white bars) or EP2-null (black bars) murine lung fibroblasts in the presence or absence of bFGF, PGE2, or butaprost. Results are expressed as the mean number of migrated cells per hpf ± SEM. Experiments were performed in triplicate wells, and the experiment was repeated twice with representative results shown (*P < 0.001).
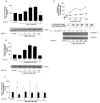
Figure 2
PGE2 and butaprost treatment of lung fibroblasts increases PTEN activity. (A ) PTEN activity in IMR-90 cells increased 40% compared with control conditions by 6 h after PGE2 treatment and continuing through 18 h (*P < 0.001). PTEN activity returned to the control level by 24 h after treatment. Total PTEN levels were unchanged throughout the time course. (B ) PTEN activity in IMR-90 cells was increased 3.5-fold after butaprost treatment as compared with control conditions (*P < 0.001). PTEN activity returned to that of control by 24 h. Total PTEN levels were unchanged throughout the time course. (C ) Increased PTEN activity is dependent on EP2 signaling. PTEN activity increases in a time-dependent fashion after butaprost treatment in C57Bl/6 cells (white bars), but not EP2-null cells (black bars). (D ) Intracellular production of cyclic AMP (cAMP) in IMR-90 cells after butaprost (triangles) or PGE2 (squares) stimulation, reported in arbitrary units. The ratio of cAMP increase after butaprost as compared with PGE2 is reported below the graph. The results are representative of two independent experiments. (E ) Butaprost treatment resulted in a time-dependent diminution in S473 phospho-Akt. Control indicates IMR-90 cells in serum-free media alone. The data are representative of two sets of lysates prepared independently. Total Akt levels were unaffected. Numbers above lanes indicate relative density of phospho-Akt to Akt.
Figure 3
PTEN activity is required for EP2-mediated inhibition of fibro-blast migration. Migration in wild-type (C57Bl/6) (white bars) murine embryonic fibroblasts and _pten_-null (black bars) embryonic fibroblasts. Baseline migration of _pten_-null fibroblasts was significantly greater than control fibroblasts. In the presence of bFGF, both control and _pten_-null fibroblasts demonstrated increased migration. Whereas bFGF-induced migration of wild-type fibroblasts was significantly inhibited by either butaprost or PGE2 (*P < 0.001), migration in _pten_-null cells could not be inhibited by either agent.
Figure 4
Butaprost treatment of lung fibroblasts results in decreased PTEN tyrosine phosphorylation. (A ) IMR-90 lung fibroblasts treated with butaprost (500 nM) were lysed, separated by electrophoresis, and were immunoblotted with antibodies against PTEN phosphorylated on S380 or S380T382T383, or total PTEN. No change in phosphorylation of these residues was observed after butaprost treatment. (B ) IMR-90 lung fibro-blasts treated with butaprost (500 nM) were lysed and PTEN was immunoprecipitated. Immunoprecipitates were separated by electrophoresis and immunoblotted with antibody against phosphotyrosine. Numbers above lanes indicate relative density of phosphotyrosine to total PTEN. Tyrosine phosphorylation of PTEN decreased over time after butaprost treatment. To ensure equal protein loading, blots were stripped and reprobed for PTEN.
Similar articles
- Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - Qualitative evidence synthesis informing our understanding of people's perceptions and experiences of targeted digital communication.
Ryan R, Hill S. Ryan R, et al. Cochrane Database Syst Rev. 2019 Oct 23;10(10):ED000141. doi: 10.1002/14651858.ED000141. Cochrane Database Syst Rev. 2019. PMID: 31643081 Free PMC article. - Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.
Lee CC, Chen CW, Yen HK, Lin YP, Lai CY, Wang JL, Groot OQ, Janssen SJ, Schwab JH, Hsu FM, Lin WH. Lee CC, et al. Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924 - Using Experience Sampling Methodology to Capture Disclosure Opportunities for Autistic Adults.
Love AMA, Edwards C, Cai RY, Gibbs V. Love AMA, et al. Autism Adulthood. 2023 Dec 1;5(4):389-400. doi: 10.1089/aut.2022.0090. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116059 Free PMC article. - Antioxidants for female subfertility.
Showell MG, Mackenzie-Proctor R, Jordan V, Hart RJ. Showell MG, et al. Cochrane Database Syst Rev. 2017 Jul 28;7(7):CD007807. doi: 10.1002/14651858.CD007807.pub3. Cochrane Database Syst Rev. 2017. PMID: 28752910 Free PMC article. Updated. Review.
Cited by
- The Lipid Portion of Activated Platelet-Rich Plasma Significantly Contributes to Its Wound Healing Properties.
Hoeferlin LA, Huynh QK, Mietla JA, Sell SA, Tucker J, Chalfant CE, Wijesinghe DS. Hoeferlin LA, et al. Adv Wound Care (New Rochelle). 2015 Feb 1;4(2):100-109. doi: 10.1089/wound.2014.0589. Adv Wound Care (New Rochelle). 2015. PMID: 25713752 Free PMC article. - Molecular determinants of mesenchymal cell activation in fibroproliferative diseases.
Penke LR, Peters-Golden M. Penke LR, et al. Cell Mol Life Sci. 2019 Nov;76(21):4179-4201. doi: 10.1007/s00018-019-03212-3. Epub 2019 Sep 28. Cell Mol Life Sci. 2019. PMID: 31563998 Free PMC article. Review. - Airway remodeling in murine asthma correlates with a defect in PGE2 synthesis by lung fibroblasts.
Stumm CL, Wettlaufer SH, Jancar S, Peters-Golden M. Stumm CL, et al. Am J Physiol Lung Cell Mol Physiol. 2011 Nov;301(5):L636-44. doi: 10.1152/ajplung.00158.2011. Epub 2011 Aug 26. Am J Physiol Lung Cell Mol Physiol. 2011. PMID: 21873451 Free PMC article. - Prostaglandin E₂ increases fibroblast gene-specific and global DNA methylation via increased DNA methyltransferase expression.
Huang SK, Scruggs AM, Donaghy J, McEachin RC, Fisher AS, Richardson BC, Peters-Golden M. Huang SK, et al. FASEB J. 2012 Sep;26(9):3703-14. doi: 10.1096/fj.11-203323. Epub 2012 May 29. FASEB J. 2012. PMID: 22645246 Free PMC article. - PGE(2) inhibition of TGF-beta1-induced myofibroblast differentiation is Smad-independent but involves cell shape and adhesion-dependent signaling.
Thomas PE, Peters-Golden M, White ES, Thannickal VJ, Moore BB. Thomas PE, et al. Am J Physiol Lung Cell Mol Physiol. 2007 Aug;293(2):L417-28. doi: 10.1152/ajplung.00489.2006. Epub 2007 Jun 8. Am J Physiol Lung Cell Mol Physiol. 2007. PMID: 17557799 Free PMC article.
References
- Clark RA. Basics of cutaneous wound repair. J Dermatol Surg Oncol. 1993;19:693–706. - PubMed
- Mutsaers SE, Bishop JE, McGrouther G, Laurent GJ. Mechanisms of tissue repair: from wound healing to fibrosis. Int J Biochem Cell Biol. 1997;29:5–17. - PubMed
- American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161:646–664. - PubMed
- White ES, Thannickal VJ, Carskadon SL, Dickie EG, Livant DL, Markwart S, Toews GB, Arenberg DA. Integrin alpha4beta1 regulates migration across basement membranes by lung fibroblasts: a role for phosphatase and tensin homologue deleted on chromosome 10. Am J Respir Crit Care Med. 2003;168:436–442. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-HL58897/HL/NHLBI NIH HHS/United States
- T32 HL007749/HL/NHLBI NIH HHS/United States
- P50 HL056402/HL/NHLBI NIH HHS/United States
- T32-HL07749/HL/NHLBI NIH HHS/United States
- K08-HL70990/HL/NHLBI NIH HHS/United States
- P50-HL56402/HL/NHLBI NIH HHS/United States
- 7R01-HL71586/HL/NHLBI NIH HHS/United States
- R01 HL058897/HL/NHLBI NIH HHS/United States
- R01 HL071586/HL/NHLBI NIH HHS/United States
- K08 HL070990/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials