Inhibition of Hsp72-mediated protein refolding by 4-hydroxy-2-nonenal - PubMed (original) (raw)
Inhibition of Hsp72-mediated protein refolding by 4-hydroxy-2-nonenal
David L Carbone et al. Chem Res Toxicol. 2004 Nov.
Abstract
A proteomic approach was applied to liver cytosol from rats fed a diet consisting of high fat and ethanol to identify 4-hydroxy-2-nonenal (4-HNE)-modified proteins in vivo. Cytosolic Hsp72, the inducible variant of the Hsp70 heat shock protein family, was consistently among the proteins modified by 4-HNE. Despite 1.3-fold induction of Hsp72 in the livers of ethanol-fed animals, no increase in Hsp70-mediated luciferase refolding in isolated heptocytes was observed, suggesting inhibition of this process by 4-HNE. A 50% and 75% reduction in luciferase refolding efficiency was observed in rabbit reticulocyte lysate (RRL) supplemented with recombinant Hsp72 which had been modified in vitro with 10 and 100 microM 4-HNE, respectively. This observation was accompanied by a 25% and 50% decrease in substrate binding by the chaperone following the same treatment; however, no effect on complex formation between Hsp72 and its co-chaperone Hsp40 was observed. Trypsin digest and mass spectral analysis of Hsp72 treated with 10 and 100 microM 4-HNE consistently identified adduct formation at Cys267 in the ATPase domain of the chaperone. The role of this residue in the observed inhibition was demonstrated through the use of DnaK, a bacterial Hsp70 variant lacking Cys267. DnaK was resistant to 4-HNE inactivation. Additionally, Hsp72 was resistant to inactivation by the thiol-unreactive aldehyde malondialdehyde (MDA), further supporting a role for Cys in Hsp72 inhibition by 4-HNE. Finally, the affinity of Hsp72 for ATP was decreased 32% and 72% following treatment of the chaperone with 10 and 100 microM 4-HNE, respectively. In a model of chronic alcoholic liver injury, induction of Hsp72 was not accompanied by an increase in protein refolding ability. This is likely the result of 4-HNE modification of the Hsp72 ATPase domain.
Figures
Figure 1
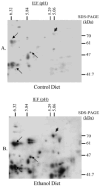
Two-dimensional electrophoresis and immunoblot against 4-HNE-modified proteins from control (A) and ethanol-fed (B) rat liver cytosolic fractions. Hsp72 was identified following in-gel digest and LC-MS/MS analysis interfaced with the MASCOT search engine, and is indicated (arrowhead) along with three spots used for alignment (arrows).
Figure 2
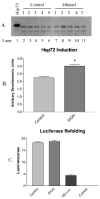
(A) Induction of Hsp72 is demonstrated in a representative immunoblot using antibodies against the chaperone in control (animals C1–C5; lanes 2–6) and ethanol-fed (animals E1–E5; lanes 7–11) rats. Protein identity was confirmed with recombinant Hsp72 (lane 1). (B) Densitometry confirms an approximate 1.3-fold induction (n = 4 ± SD). (C) Protein refolding by lysed hepatocytes from control and ethanol-fed animals using heat-denatured luciferase, with controls for spontaneous refolding (HD Luc.) and intrinsic luminescence (cytosol) (n = 3 ± SEM).
Figure 3
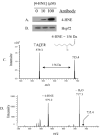
In vitro modification of recombinant Hsp72 by 4-HNE (A), and confirmation of consistent gel loading using antibodies against Hsp72 (B). Identification of 4-HNE adduct location on the Hsp72 peptide TACER, corresponding to amino acids 265–269. The peaks at m/z 579.2 and 735.4 represent the unmodified and 4HNE-modified peptide, respectively (C). Confirmation of Cys267 modification by 4-HNE was acquired fromMS/MS analysis of the peak at m/z 735.4, clearly demonstrating neutral loss of the 156 D 4-HNE adduct and fragmentation consistent with modification of Cys267 (D).
Figure 4
(A) Refolding of heat-denatured recombinant firefly luciferase by rabbit reticulocyte lysate alone (RRL control) or supplemented with Hsp40 and 4-HNE untreated (0 _μ_M) or treated (10 _μ_M, 100 _μ_M) Hsp72. Inhibition of refolding correlates to 4-HNE pretreatment, and decreases refolding below that of the RRL control (n = 3 ± SD, * indicates significant difference from all groups). (B) Use of the proteasome inhibitor MG-132 has no effect on the recovery of luciferase activity, ruling out enhanced interaction between Hsp72 and the 26S proteasome. Free 4-HNE demonstrates inhibition of luciferase refolding, but is less pronounced than that following pretreatment of Hsp72 (B) (n = 3 ± SD, * indicates significant difference from all groups).
Figure 5
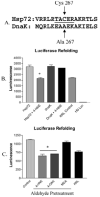
(A) CLUSTAL W amino acid alignment of Hsp72 (P08107) and bacterial DnaK (P04475), in which Cys267 is replaced with Ala. The peptide obtained from trypsin digest of Hsp72 is underlined. (B) Refolding of heat-denatured luciferase by rabbit reticulocyte lysate supplemented with control or 4-HNE-treated Hsp72 or DnaK, demonstrating resistance of DnaK to 4-HNE inhibition (n = 3 ± SEM). (C) Refolding of heat-denatured luciferase by rabbit reticulocyte lysate supplemented with Hsp72 pretreated with no aldehyde (control), Cys-reactive aldehydes (4-HNE, 4-ONE), or Cys nonreactive aldehyde (MDA), demonstrating a crucial role for Cys in aldehyde-mediated Hsp72 inhibition (n = 3 ± SEM).
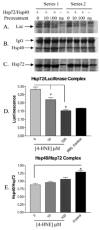
Figure 6
Co-immunoprecipitation of Hsp72 from rabbit reticulocyte lysate supplemented with Hsp40 and 4-HNE treated (10 _μ_M, 100 _μ_M) or untreated (0 _μ_M) Hsp72 from a series of two independent experiments. Panels A–C demonstrate immunoreactivity against substrate (heat-denatured luciferase), co-chaperone (Hsp40), and Hsp72. (D) A decrease in substrate binding by 4-HNE pretreated Hsp72 is clearly demonstrated when luciferase signal is normalized to Hsp72 signal (n = 2 ± SD). (E) No change in complex formation between 4-HNE pretreated Hsp72 and Hsp40 following normalization of Hsp40 signal to Hsp72 signal (n = 2 ± SD).
Figure 7
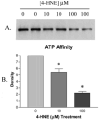
(A) Decreased affinity of 4-HNE pretreated Hsp72 for ATP following purification with ATP-linked agarose beads is demonstrated by immunoblot against Hsp72 and is confirmed by densitometry in panel B (n = 2 ± SD).
Similar articles
- Modification of heat shock protein 90 by 4-hydroxynonenal in a rat model of chronic alcoholic liver disease.
Carbone DL, Doorn JA, Kiebler Z, Ickes BR, Petersen DR. Carbone DL, et al. J Pharmacol Exp Ther. 2005 Oct;315(1):8-15. doi: 10.1124/jpet.105.088088. Epub 2005 Jun 10. J Pharmacol Exp Ther. 2005. PMID: 15951401 - Cysteine modification by lipid peroxidation products inhibits protein disulfide isomerase.
Carbone DL, Doorn JA, Kiebler Z, Petersen DR. Carbone DL, et al. Chem Res Toxicol. 2005 Aug;18(8):1324-31. doi: 10.1021/tx050078z. Chem Res Toxicol. 2005. PMID: 16097806 - Heat-shock preconditioning reduces oxidative protein denaturation and ameliorates liver injury by carbon tetrachloride in rats.
Yamamoto H, Yamamoto Y, Yamagami K, Kume M, Kimoto S, Toyokuni S, Uchida K, Fukumoto M, Yamaoka Y. Yamamoto H, et al. Res Exp Med (Berl). 2000 Jun;199(6):309-18. doi: 10.1007/s004339900040. Res Exp Med (Berl). 2000. PMID: 10945649 - Overview of lipid peroxidation products and hepatic protein modification in alcoholic liver disease.
Smathers RL, Galligan JJ, Stewart BJ, Petersen DR. Smathers RL, et al. Chem Biol Interact. 2011 Jun 30;192(1-2):107-12. doi: 10.1016/j.cbi.2011.02.021. Epub 2011 Feb 24. Chem Biol Interact. 2011. PMID: 21354120 Free PMC article. Review. - Molecular mechanisms of 4-hydroxy-2-nonenal and acrolein toxicity: nucleophilic targets and adduct formation.
LoPachin RM, Gavin T, Petersen DR, Barber DS. LoPachin RM, et al. Chem Res Toxicol. 2009 Sep;22(9):1499-508. doi: 10.1021/tx900147g. Chem Res Toxicol. 2009. PMID: 19610654 Free PMC article. Review.
Cited by
- Exploring the biology of lipid peroxidation-derived protein carbonylation.
Fritz KS, Petersen DR. Fritz KS, et al. Chem Res Toxicol. 2011 Sep 19;24(9):1411-9. doi: 10.1021/tx200169n. Epub 2011 Aug 18. Chem Res Toxicol. 2011. PMID: 21812433 Free PMC article. Review. - The yeast Hsp70 Ssa1 is a sensor for activation of the heat shock response by thiol-reactive compounds.
Wang Y, Gibney PA, West JD, Morano KA. Wang Y, et al. Mol Biol Cell. 2012 Sep;23(17):3290-8. doi: 10.1091/mbc.E12-06-0447. Epub 2012 Jul 18. Mol Biol Cell. 2012. PMID: 22809627 Free PMC article. - 4-Hydroxy-Trans-2-Nonenal in the Regulation of Anti-Oxidative and Pro-Inflammatory Signaling Pathways.
Sonowal H, Ramana KV. Sonowal H, et al. Oxid Med Cell Longev. 2019 Nov 6;2019:5937326. doi: 10.1155/2019/5937326. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31781341 Free PMC article. Review. - Regulation of stress signaling pathways by protein lipoxidation.
Patinen T, Adinolfi S, Cortés CC, Härkönen J, Jawahar Deen A, Levonen AL. Patinen T, et al. Redox Biol. 2019 May;23:101114. doi: 10.1016/j.redox.2019.101114. Epub 2019 Jan 16. Redox Biol. 2019. PMID: 30709792 Free PMC article. Review. - Molecular Changes Induced by Oxidative Stress that Impair Human Sperm Motility.
Nowicka-Bauer K, Nixon B. Nowicka-Bauer K, et al. Antioxidants (Basel). 2020 Feb 4;9(2):134. doi: 10.3390/antiox9020134. Antioxidants (Basel). 2020. PMID: 32033035 Free PMC article. Review.
References
- Young JC, Hoogenraad NJ, Hartl FU. Molecular chaperones Hsp90 and Hsp70 deliver preproteins to the mitochondrial import receptor Tom70. Cell. 2003;112:41–50. - PubMed
- Fink AL. Chaperone-mediated protein folding. Physiol Rev. 1999;79:425–442. - PubMed
- Bercovich B, Stancovski I, Mayer A, Blumenfeld N, Laszlo A, Schwartz AL, Ciechanover A. Ubiquitin-dependent degradation of certain protein substrates in vitro requires the molecular chaperone Hsc70. J Biol Chem. 1997;272:9002–9010. - PubMed
- Goasduff T, Cederbaum AI. CYP2E1 degradation by in vitro reconstituted systems: role of the molecular chaperone Hsp90. Arch Biochem Biophys. 2000;379:321–330. - PubMed
- Glover JR, Lindquist S. Hsp104, Hsp70, and Hsp40: a novel chaperone system that rescues previously aggregated proteins. Cell. 1998;94:73–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 ES11937/ES/NIEHS NIH HHS/United States
- R01AA09300/AA/NIAAA NIH HHS/United States
- F31 AA014308/AA/NIAAA NIH HHS/United States
- R01ES09410/ES/NIEHS NIH HHS/United States
- R01 ES009410/ES/NIEHS NIH HHS/United States
- F32 ES011937/ES/NIEHS NIH HHS/United States
- R01 AA009300/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources