Interleukin-8/CXCL8 is a growth factor for human lung cancer cells - PubMed (original) (raw)
Interleukin-8/CXCL8 is a growth factor for human lung cancer cells
Y M Zhu et al. Br J Cancer. 2004.
Abstract
Interleukin-8/CXCL8 (IL-8) is a chemokine and angiogenic factor. Recently, IL-8 was identified as an autocrine growth factor in several human cancers. Here, we investigated the expression and function of IL-8 in lung cancer cells. The expressions of IL-8 and its receptors, CXCR1 and CXCR2, were examined in a panel of non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) cell lines. Using reverse transcription-polymerase chain reaction (RT-PCR) and enzyme-linked immunosorbent assay, we found that all NSCLC cell lines tested produced modest or high levels of IL-8 (up to 51 ng ml(-1) 10(6) cells(-1)). Expression of CXCR1 and CXCR2 was found by RT-PCR and flow cytometry in two out of three cell lines. In contrast, SCLC cell lines produced very low or undetectable levels of IL-8, but expressed CXCR1 and CXCR2. We next investigated whether IL-8 could act as an autocrine growth factor in two NSCLC cell lines (H460 and MOR/P) expressing both IL-8 and its receptors. We found that cell proliferation was attenuated by anti-IL-8 neutralising antibody to 71 and 76% in H460 and MOR/P, respectively (P<0.05). Exogenous IL-8 significantly stimulated cell proliferation in four SCLC cell lines tested in a dose-dependent fashion. Cell proliferation was increased by between 18% (P<0.05) and 37% (P<0.05). Stimulation of cell proliferation by IL-8 was also demonstrated by analysis of proliferating cell nuclear antigen expression and cell cycle in H69 cells. Furthermore, we investigated which receptor(s) mediated the mitogenic function of IL-8 in lung cancer cells. We found that cell proliferation was significantly reduced by anti-CXCR1 antibody but not by anti-CXCR2 antibody. In conclusion, IL-8 can act as an autocrine and/or paracrine growth factor for lung cancer cells, and the mitogenic function of IL-8 in lung cancer is mediated mainly by CXCR1 receptor.
Figures
Figure 1
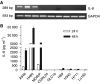
Expression of IL-8 mRNA and protein in lung cancer cell lines. Expression of IL-8 mRNA was measured by RT–PCR (A). A 1 _μ_g portion of total RNA was reverse-transcribed for PCR reactions of IL-8 and control GAPDH. The expected 289 bp band of IL-8 mRNA was strongly expressed in A549, H460 and MOR/P, but was undetectable in all SCLC cell lines. Production of IL-8 protein was measured by ELISA (B). Conditioned medium was collected after 1 × 106 cells were cultured in serum-free RPMI medium for 48 h. Each bar is the mean±s.e. of three determinations from two independent experiments.
Figure 2
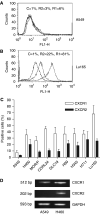
Expression of CXCR1 and CXCR2 in lung cancer cell lines. Expression of CXCR1 and CXCR2 proteins on the cell surface was measured by flow cytometry with mouse anti-human CXCR1 (R1) and CXCR2 (R2) and mouse IgG as control (C). Representative flow cytometric histograms of A549 and Lu165 showing the low expressions of CXCR1 and CXCR2 in A549 (A) and high expressions of CXCR1 and CXCR2 in Lu165 (B), respectively. Percentage of positive cells of CXCR1 and CXCR2 in nine lung cancer cell lines are summarised in (C). Each bar is the mean±s.e. of four independent experiments. Expression of CXCR1 and CXCR2 mRNA was measured by RT–PCR in A549 and H460 (as a positive control) (D). Total RNA (1.5 _μ_g) was reverse-transcribed for PCR reactions of CXCR1, CXCR2 and control GAPDH. The expected 512 bp band of CXCR1 was expressed in A549 and H460. The expected 202 bp band of CXCR2 was expressed in control cell H460 but not in A549 cells.
Figure 3
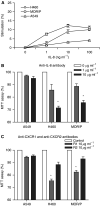
Interleukin-8 is an autocrine growth factor in H460 and MOR/P cells. (A) Non-small cell lung cancer cells were treated with rIL-8 (at a concentration of 0, 1, 10 and 100 ng ml−1) for 48 h. Cell proliferation was measured by MTT assay. Each point is the mean±s.e. of three determinations from two independent experiments. (B) Non-small cell lung cancer cells were treated with anti-IL-8 neutralising antibody (1 or 10 _μ_g ml−1) for 48 h. Cell proliferation was measured by MTT assay. Each bar is the mean±s.e. of three determinations from two independent experiments. *P<0.05. (C) Non-small cell lung cancer cells were treated with anti-CXCR1 or anti-CXCR2 neutralising antibody (10 _μ_g ml−1) or control antibody (mouse IgG at 10 _μ_g ml−1) for 48 h. Cell proliferation was measured by MTT assay. Each bar is the mean±s.e. of three determinations from two independent experiments. *P<0.05.
Figure 4
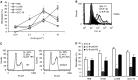
Recombinant IL-8 stimulates SCLC cell proliferation through CXCR1. (A) The SCLC cell lines H69, Lu165, H345 and GLC-19 were treated with rIL-8 at concentrations of 0.01, 0.1, 1 and 10 ng ml−1 for 48 h. Cell proliferation was measured by MTT assay. Each point is the mean±s.e. of three determinations from two independent experiments. *P<0.05. (B) Detection of PCNA intensity by flow cytometry after H69 cells was treated with rIL-8 (1 ng ml−1) for 48 h. (C) Analysis of DNA content by flow cytometry after H69 cells was treated with rIL-8 (1 ng ml−1). (D) Small cell lung cancer cells were treated with rIL-8 plus anti-CXCR1 or anti-CXCR2 antibody for 48 h. The concentration of rIL-8 for each cell line was chosen for maximal stimulation: 10 ng ml−1 for Lu165 and 1 ng ml−1 for others. The final concentration of anti-CXCR1 or anti-CXCR2 was 10 _μ_g ml−1. Each bar is the mean±s.e. of three independent determinations of two experiments. *P<0.05 for inhibition of rIL-8-induced proliferation by anti-CXCR1 antibody.
Similar articles
- Expression of interleukin 8 and its receptors in human colon carcinoma cells with different metastatic potentials.
Li A, Varney ML, Singh RK. Li A, et al. Clin Cancer Res. 2001 Oct;7(10):3298-304. Clin Cancer Res. 2001. PMID: 11595728 - The activation of IL-8 receptors in cultured guinea pig Müller glial cells is modified by signals from retinal pigment epithelium.
Malgorzata Goczalik I, Raap M, Weick M, Milenkovic I, Heidmann J, Enzmann V, Wiedemann P, Reichenbach A, Francke M. Malgorzata Goczalik I, et al. J Neuroimmunol. 2005 Apr;161(1-2):49-60. doi: 10.1016/j.jneuroim.2004.12.004. J Neuroimmunol. 2005. PMID: 15748943 - Autocrine role of interleukin-8 in induction of endothelial cell proliferation, survival, migration and MMP-2 production and angiogenesis.
Li A, Varney ML, Valasek J, Godfrey M, Dave BJ, Singh RK. Li A, et al. Angiogenesis. 2005;8(1):63-71. doi: 10.1007/s10456-005-5208-4. Angiogenesis. 2005. PMID: 16132619 - Mitogenic effects of interleukin-8/CXCL8 on cancer cells.
Zhu YM, Woll PJ. Zhu YM, et al. Future Oncol. 2005 Oct;1(5):699-704. doi: 10.2217/14796694.1.5.699. Future Oncol. 2005. PMID: 16556047 Review. - The CXCL8-CXCR1/2 pathways in cancer.
Liu Q, Li A, Tian Y, Wu JD, Liu Y, Li T, Chen Y, Han X, Wu K. Liu Q, et al. Cytokine Growth Factor Rev. 2016 Oct;31:61-71. doi: 10.1016/j.cytogfr.2016.08.002. Epub 2016 Aug 25. Cytokine Growth Factor Rev. 2016. PMID: 27578214 Free PMC article. Review.
Cited by
- Proteomic-Based Approaches for the Study of Cytokines in Lung Cancer.
Marrugal Á, Ojeda L, Paz-Ares L, Molina-Pinelo S, Ferrer I. Marrugal Á, et al. Dis Markers. 2016;2016:2138627. doi: 10.1155/2016/2138627. Epub 2016 Jun 30. Dis Markers. 2016. PMID: 27445423 Free PMC article. Review. - CCR9/CCL25 expression in non-small cell lung cancer correlates with aggressive disease and mediates key steps of metastasis.
Gupta P, Sharma PK, Mir H, Singh R, Singh N, Kloecker GH, Lillard JW Jr, Singh S. Gupta P, et al. Oncotarget. 2014 Oct 30;5(20):10170-9. doi: 10.18632/oncotarget.2526. Oncotarget. 2014. PMID: 25296976 Free PMC article. - Interleukin-8/CXCR1 Signaling Contributes to the Progression of Pulmonary Adenocarcinoma Resulting in Malignant Pleural Effusion.
Chang YM, Huang WY, Yang SH, Jan CI, Nieh S, Lin YS, Chen SF, Lin YC. Chang YM, et al. Cells. 2024 Jun 3;13(11):968. doi: 10.3390/cells13110968. Cells. 2024. PMID: 38891100 Free PMC article. - Innate Immune Landscape in Early Lung Adenocarcinoma by Paired Single-Cell Analyses.
Lavin Y, Kobayashi S, Leader A, Amir ED, Elefant N, Bigenwald C, Remark R, Sweeney R, Becker CD, Levine JH, Meinhof K, Chow A, Kim-Shulze S, Wolf A, Medaglia C, Li H, Rytlewski JA, Emerson RO, Solovyov A, Greenbaum BD, Sanders C, Vignali M, Beasley MB, Flores R, Gnjatic S, Pe'er D, Rahman A, Amit I, Merad M. Lavin Y, et al. Cell. 2017 May 4;169(4):750-765.e17. doi: 10.1016/j.cell.2017.04.014. Cell. 2017. PMID: 28475900 Free PMC article. - Immune checkpoint blockade induced shifts in cytokine expression patterns in peripheral blood of head and neck cancer patients are linked to outcome.
Röhl L, Wellhausen J, Berszin M, Krücken I, Zebralla V, Pirlich M, Wiegand S, Dietz A, Wald T, Wichmann G. Röhl L, et al. Front Immunol. 2023 Oct 2;14:1237623. doi: 10.3389/fimmu.2023.1237623. eCollection 2023. Front Immunol. 2023. PMID: 37849764 Free PMC article.
References
- Addison CL, Daniel TO, Burdick MD, Liu H, Ehlert JE, Xue YY, Buechi L, Walz A, Richmond A, Strieter RM (2000) The CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR+ CXC chemokine-induced angiogenic activity. J Immunol 165: 5269–5277 - PubMed
- Anderson IC, Mari SE, Broderick RJ, Mari BP, Shipp MA (2000) The angiogenic factor interleukin 8 is induced in non-small cell lung cancer/pulmonary fibroblast cocultures. Cancer Res 60: 269–272 - PubMed
- Brew R, Erikson JS, West DC, Kinsella AR, Slavin J, Christmas SE (2000) Interleukin-8 as an autocrine growth factor for human colon carcinoma cells in vitro. Cytokine 12: 78–85 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical