Cdc42 mediates nucleus movement and MTOC polarization in Swiss 3T3 fibroblasts under mechanical shear stress - PubMed (original) (raw)
Cdc42 mediates nucleus movement and MTOC polarization in Swiss 3T3 fibroblasts under mechanical shear stress
Jerry S H Lee et al. Mol Biol Cell. 2005 Feb.
Abstract
Nucleus movement is essential during nucleus positioning for tissue growth and development in eukaryotic cells. However, molecular regulators of nucleus movement in interphase fibroblasts have yet to be identified. Here, we report that nuclei of Swiss 3T3 fibroblasts undergo enhanced movement when subjected to shear flows. Such movement includes both rotation and translocation and is dependent on microtubule, not F-actin, structure. Through inactivation of Rho GTPases, well-known mediators of cytoskeleton reorganization, we demonstrate that Cdc42, not RhoA or Rac1, controls the extent of nucleus translocation, and more importantly, of nucleus rotation in the cytoplasm. In addition to generating nuclei movement, we find that shear flows also causes repositioning of the MTOC in the direction of flow. This behavior is also controlled by Cdc42 via the Par6/protein kinase Czeta pathway. These results are the first to establish Cdc42 as a molecular regulator of not only shear-induced MTOC polarization in Swiss 3T3 fibroblasts, but also of shear-induced microtubule-dependent nucleus movement. We propose that the movements of MTOC and nucleus are coupled chemically, because they are both regulated by Cdc42 and dependent on microtubule structure, and physically, possibly via Hook/SUN family homologues similar to those found in Caenorhabditis elegans.
Figures
Figure 5.
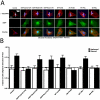
The MTOC of sheared fibroblasts is polarized and its location with respect to the nucleus is controlled by Cdc42. (A) MTOC location in Swiss 3T3 fibroblasts with respect to the nucleus and the shear flow direction. Cells were either transfected with EGFP-tagged constructs (EGFP:Cdc42T17N, EGFP:RhoAT19N, EGFP:Rac1T17N) or a combination of protein plasmid (WT Par6B, ΔNt Par6B, WT PKCζ, DN PKCζ) and blank EGFP vector. Proteins were given 24 h to express and positive-transfected fibroblasts exhibited green fluorescence. Fibroblasts were subjected to 40 min of shear flow (τw = 9.4 dyn/cm2) and then fixed and stained for MT and nuclear DNA using α-tubulin/Alexa568 and DAPI, respectively. The nucleus was divided into two zones, and MTOC location was determined based on this separation. Bar, 20 μm. (B) MTOC polarization in fibroblasts is controlled by Cdc42, not RhoA or Rac1. Shear-induced MTOC polarization is inhibited by Cdc42 inactivation and is unaffected by RhoA and Rac1 inactivation. Transfection of EGFP: Cdc42T17N abrogates shear-induced MTOC polarization in Swiss 3T3 fibroblasts. Transfections of EGFP:RhoAT19N and EGFP: Rac1T17N did not alter distribution of MTOC location from unsheared transfected cells. Dominant negative/kinase inactive Par6/PKCζ transfection abolished MTOC polarization, similar to fibroblasts transfected with EGFP:Cdc42T17N. Transfection of wild-type Par6/PKCζ did not alter shear-induced polarization in the cells (n = 75 for all conditions).
Figure 1.
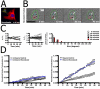
External mechanical shear stress enhances rigid-body movements of the nucleus in Swiss 3T3 fibroblasts. (A) Time-lapse phase contrast sequence of a Swiss 3T3 fibroblast subjected to shear flow for 40 min (shear stress 9.4 dyn/cm2). In the first frame, the solid red circle indicates the initial position and shape of the nucleus. For reference, subsequent dashed red circles mark the initial position of the nucleus. The green circles indicate the instantaneous position and shape of the nucleus. Bar, 20 μm. (B) Net displacement of the nucleus (corrected for cell displacement) in the sheared fibroblast shown in A, using time-dependent coordinates of the nucleus centroid (xn, yn) and the cell centroid (xc, yc). (C) Normalized nucleus translocation (δ) of sheared cells (•, n = 7) and unsheared control cells (○, n = 5). (D) Schematic for the computation of nucleus shape factor σ (using both the apparent area, A, and perimeter, P, of the nucleus), nucleus-nucleolus interdistance λ, and nucleus rotation θ. Both λ and θ are computed using centroid positions of the nucleus and nucleoli as indicated by white dots (see Materials and Methods). (E) Changes in nucleus shape factor σ and nucleus-nucleolus interdistances λ normalized by their respective initial values, σo and λo, and distribution of nucleus rotation (θ) in control sheared fibroblasts (n = 7).
Figure 2.
Cell treatment with the F-actin depolymerizing agent latrunculin-B causes no change in the motion of the nucleus. (A) F-actin organization in Swiss 3T3 fibroblasts treated with varying concentrations of F-actin depolymerizing agent, latrunculin-B (LA-B), for 30 min. F-actin and nuclear DNA were visualized using Alexa-488 phallodin and DAPI, respectively. F-actin depolymerization in the perinuclear region is evident for all tested LA-B concentrations. Significant F-actin depolymerization in the lamella did not occur until 315 nM. Bar, 20 μm. (B) Normalized nucleus centroid translocation of sheared control cells (black, n = 7) and LA-B–treated fibroblasts (315 nM, yellow, n = 3).
Figure 3.
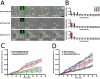
The movement of the nucleus depends on the integrity of microtubules. (A) MT organization in fibroblasts treated for 30 min with 1 μg/ml nocodazole. Immunofluorescence of MT, nuclear DNA, and F-actin showed completely depolymerized MT structure with intact F-actin structure (inset). (B) Time-lapse phase contrast sequence of sheared fibroblast treated with nocodazole reveals a highly ductile nucleus. Solid and dashed colored circles indicate the initial position of the nucleus (green) and the initial positions of nucleoli (cyan and red); white arrows show the instantaneous location of the nucleoli. Bar, 20 μm. (C) Changes in nucleus shape factor σ and nucleus-nucleolus interdistances λ, normalized by their respective initial values, σo and λo, and distribution of nucleus rotation θ in nocodazole-treated sheared fibroblasts (n = 3). Wider distributions of σ/σo and λ/λo indicate changes in nucleus integrity, as well as nonconcerted movement of the nucleus. (D) Left: nucleus translocation in unsheared control cells (n = 5) and nocodazole-treated cells (n = 3). Right: nucleus translocation in sheared control cells (n = 7) and nocodazole-treated cells (n = 3).
Figure 4.
Dominant negative Cdc42 causes sustained nucleus rotation and enhanced nucleus translocation in fibroblasts under shear stress. (A) Time-lapse phase contrast sequence of sheared fibroblasts transfected with either EGFP:Cdc42T17N (first row), EGFP:RhoAT19N (second row), or EGFP:Rac1T17N (third row). EGFP fluorescence indicates that cells are positively transfected (inset). Solid and dashed lines show the initial position and shape of the nucleus (green) and nucleoli (red and cyan); white arrows indicate the instantaneous location of nucleoli. Bar, 20 μm. (B) Distribution of nucleus rotations in sheared cells transfected with either EGFP:Cdc4217N (first row, n = 5), EGFP:RhoAT19N (second row, n = 3), or EGFP:Rac1T17N (third row, n = 3). Final rotation distributions are shown in red. (C) Normalized nucleus translocation in sheared cells transfected with either EGFP:Cdc42T17N (red, n = 5), EGFP:RhoAT19N (yellow, n = 3), or EGFP:Rac1T17N (green, n = 3). (D) Comparison of normalized nucleus translocation of sheared control cells (black, n = 7), in cells treated with nocodazole (blue, n = 3), and cells transfected with EGFP:Cdc42T17N (red, n = 5). Translocations of nuclei in EGFP:Cdc42T17N cells is comparable to control nuclei at early times (t <15 min), but becomes greater afterward, moving nearly as much as nocodazole-treated nuclei.
Figure 6.
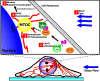
Proposed model for molecular regulation of nucleus movement and MTOC repositioning in Swiss 3T3 fibroblasts under mechanical shear. Shear flow (1) activates Cdc42, which then complexes as Cdc42-GTP to Par6 and PKCζ (2). Subsequent downstream MT reorganization due to Cdc42/Par6/PKCζ complex (3) results in MTOC reorientation toward the direction of flow inside Swiss 3T3 fibroblast (4). A physical link between the nucleus and the MTOC, possibly via Hook/SUN family proteins, causes the observed translocation and rotation of the nucleus (5).
Similar articles
- Localized cdc42 activation, detected using a novel assay, mediates microtubule organizing center positioning in endothelial cells in response to fluid shear stress.
Tzima E, Kiosses WB, del Pozo MA, Schwartz MA. Tzima E, et al. J Biol Chem. 2003 Aug 15;278(33):31020-3. doi: 10.1074/jbc.M301179200. Epub 2003 May 16. J Biol Chem. 2003. PMID: 12754216 - Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells.
Gomes ER, Jani S, Gundersen GG. Gomes ER, et al. Cell. 2005 May 6;121(3):451-63. doi: 10.1016/j.cell.2005.02.022. Cell. 2005. PMID: 15882626 - Cdc42, dynein, and dynactin regulate MTOC reorientation independent of Rho-regulated microtubule stabilization.
Palazzo AF, Joseph HL, Chen YJ, Dujardin DL, Alberts AS, Pfister KK, Vallee RB, Gundersen GG. Palazzo AF, et al. Curr Biol. 2001 Oct 2;11(19):1536-41. doi: 10.1016/s0960-9822(01)00475-4. Curr Biol. 2001. PMID: 11591323 - Mechanisms of nuclear positioning.
Reinsch S, Gönczy P. Reinsch S, et al. J Cell Sci. 1998 Aug;111 ( Pt 16):2283-95. doi: 10.1242/jcs.111.16.2283. J Cell Sci. 1998. PMID: 9683624 Review. - Nuclear positioning in migrating fibroblasts.
Zhu R, Liu C, Gundersen GG. Zhu R, et al. Semin Cell Dev Biol. 2018 Oct;82:41-50. doi: 10.1016/j.semcdb.2017.11.006. Epub 2017 Dec 11. Semin Cell Dev Biol. 2018. PMID: 29241691 Free PMC article. Review.
Cited by
- The distinct roles of the nucleus and nucleus-cytoskeleton connections in three-dimensional cell migration.
Khatau SB, Bloom RJ, Bajpai S, Razafsky D, Zang S, Giri A, Wu PH, Marchand J, Celedon A, Hale CM, Sun SX, Hodzic D, Wirtz D. Khatau SB, et al. Sci Rep. 2012;2:488. doi: 10.1038/srep00488. Epub 2012 Jul 3. Sci Rep. 2012. PMID: 22761994 Free PMC article. - Recapitulation of molecular regulators of nuclear motion during cell migration.
Sneider A, Hah J, Wirtz D, Kim DH. Sneider A, et al. Cell Adh Migr. 2019 Dec;13(1):50-62. doi: 10.1080/19336918.2018.1506654. Epub 2018 Sep 27. Cell Adh Migr. 2019. PMID: 30261154 Free PMC article. Review. - Effects of mechanical force on cytoskeleton structure and calpain-induced apoptosis in rat dorsal root ganglion neurons in vitro.
Ye Z, Wang Y, Quan X, Li J, Hu X, Huang J, Luo Z. Ye Z, et al. PLoS One. 2012;7(12):e52183. doi: 10.1371/journal.pone.0052183. Epub 2012 Dec 20. PLoS One. 2012. PMID: 23284927 Free PMC article. - Tight coupling between nucleus and cell migration through the perinuclear actin cap.
Kim DH, Cho S, Wirtz D. Kim DH, et al. J Cell Sci. 2014 Jun 1;127(Pt 11):2528-41. doi: 10.1242/jcs.144345. Epub 2014 Mar 17. J Cell Sci. 2014. PMID: 24639463 Free PMC article. - Nuclear envelope in nuclear positioning and cell migration.
Razafsky D, Wirtz D, Hodzic D. Razafsky D, et al. Adv Exp Med Biol. 2014;773:471-90. doi: 10.1007/978-1-4899-8032-8_21. Adv Exp Med Biol. 2014. PMID: 24563361 Free PMC article. Review.
References
- Allan, V. J., Thompson, H. M., and McNiven, M. A. (2002). Motoring around the Golgi. Nat. Cell Biol. 4, E236-E242. - PubMed
- Bandyopadhyay, G., Standaert, M. L., Kikkawa, U., Ono, Y., Moscat, J., and Farese, R. V. (1999). Effects of transiently expressed atypical (zeta, lambda), conventional (alpha, beta) and novel (delta, epsilon) PKC isoforms on insulin-stimulated translocation of epitope-tagged GLUT4 glucose transporters in rat adipocytes: specific interchangeable effects of protein kinases C-zeta and C-lambda. Biochem. J. 337(Pt 3), 461-470. - PMC - PubMed
- Bandyopadhyay, G., Standaert, M. L., Zhao, L., Yu, B., Avignon, A., Galloway, L., Karnam, P., Moscat, J., and Farese, R. V. (1997). Activation of PKC (alpha, beta, and zeta) by insulin in 3T3/L1 cells. Transfection studies suggest a role for PKC-zeta in glucose transport. J. Biol. Chem. 272, 2551-2558. - PubMed
- Bershadsky, A. D., Gluck, U., Denisenko, O. N., Sklyarova, T. V., Spector, I., and Ben-Ze'ev, A. (1995). The state of actin assembly regulates actin and vinculin expression by a feedback loop. J. Cell Sci. 108(Pt 3), 1183-1193. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous