Clonal type I interferon-producing and dendritic cell precursors are contained in both human lymphoid and myeloid progenitor populations - PubMed (original) (raw)
Clonal type I interferon-producing and dendritic cell precursors are contained in both human lymphoid and myeloid progenitor populations
Laurie Chicha et al. J Exp Med. 2004.
Abstract
Because of different cytokine responsiveness, surface receptor, and transcription factor expression, human CD11c(-) natural type I interferon-producing cells (IPCs) and CD11c(+) dendritic cells were thought to derive through lymphoid and myeloid hematopoietic developmental pathways, respectively. To directly test this hypothesis, we used an in vitro assay allowing simultaneous IPC, dendritic cell, and B cell development and we tested lymphoid and myeloid committed hematopoietic progenitor cells for their developmental capacity. Lymphoid and common myeloid and granulocyte/macrophage progenitors were capable of developing into both functional IPCs, expressing gene transcripts thought to be associated with lymphoid lineage development, and into dendritic cells. However, clonal progenitors for both populations were about fivefold more frequent within myeloid committed progenitor cells. Thus, in humans as in mice, natural IPC and dendritic cell development robustly segregates with myeloid differentiation. This would fit with natural interferon type I-producing cell and dendritic cell activity in innate immunity, the evolutionary older arm of the cellular immune system.
Figures
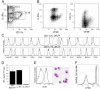
Figure 1.
Lin−CD34+IL3Rαlo cells develop in vitro to functional IPCs and immature DCs. (A) Sorted lin−CD34+IL3Rαlo cells develop to both CD11c−IL3Rαhi and CD11c+IL3Rαlo cells within 13 d and (B) five to six divisions of input cells as determined by CFSE dilution in flt3L-supplemented Ac6 stroma cell cultures. Percentages of gated populations are indicated in contour plots. (C) CD11c−IL3Rαhi and CD11c+IL3Rαlo cells express IPC- and DC-associated surface markers (bold lines) on indicated cell populations as gated in A. (D) Influenza-stimulated CD11c−IL3Rαhi cells but not CD11c+/−IL3Rαlo/− cells from culture produce similar amounts of IFNα as peripheral blood BDCA-4+ IPCs. Mean and standard deviation of 10 (cultured cells) and 5 (BDCA-4+ cells) experiments are shown. (E) LPS-activated CD11c+IL3Rαlo cells up-regulate CD80 (bold line), show typical DC morphology, and (F) are potent stimulators of allogeneic CD4+ T cells as determined by CFSE dilution at day 7. Contour plot shows overlay of CD3+ gated T cell proliferation in response to activated CD11c+IL3Rαlo cells (bold line), CD14+ monocyte-derived dendritic cells (thin line), and CD11c− cells (dashed line). Cells were plated in a 1:10 stimulator to responder cell ratio. Data are representative of two experiments.
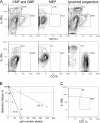
Figure 2.
Lymphoid progenitors, CMPs, and GMPs develop in vitro to both IPCs and immature DCs and contain clonal progenitors for both populations. (A) Lymphoid progenitors, CMPs, and GMPs generate comparable relative numbers of both CD11c−IL3Rαhi and CD11c+ IL3Rαlo cells, whereas MEPs generate few, if any, CD11c−IL3Rαhi and no CD11c+IL3Rαlo cells (top). Only lymphoid progenitors developed to CD19+ B cells (bottom). Representative contour plots of three experiments analyzed at day 14 of culture. (B) Frequencies of myeloid (solid line) and lymphoid (dashed line) progenitors simultaneously differentiating to IPCs and DCs were determined by limiting dilution. The x axis depicts the number of plated cells and the y axis detection failure for combined IPC and DC read-out. Horizontal and vertical bars mark the 37% negative read-out predicting progenitor frequencies (numbers in brackets). Statistics were calculated on the basis of mean values of each dilution step from three and six independent experiments for myeloid and lymphoid progenitors, respectively. Correlation coefficients for curve extrapolations were r = 0.9372 and r = 0.93996 for myeloid and lymphoid progenitors, respectively. (C) Dot plot shows typical combined IPC and DC read-out from a single myeloid progenitor at day 14 d of culture. Percentages of gated populations are indicated in all plots.
Figure 3.
Gene expression in myeloid and lymphoid progenitors and respective progenitor-derived IPCs and DCs Expression of indicated mRNA gene transcripts in progenitors and in offspring day 14 IPCs and DCs are shown in arbitrary units relative to endogenous β-actin. Data are representative of three independent experiments. c-mpl, thrombopoietin receptor; flt3, flt3 receptor; pTα, pre–T cell receptor α; IL-7Rα: IL-7 receptor α; spi-B, Spi-B transcription factor; TLR, Toll-like receptor.
Similar articles
- Developmental origin of interferon-alpha-producing dendritic cells from hematopoietic precursors.
Karsunky H, Merad M, Mende I, Manz MG, Engleman EG, Weissman IL. Karsunky H, et al. Exp Hematol. 2005 Feb;33(2):173-81. doi: 10.1016/j.exphem.2004.10.010. Exp Hematol. 2005. PMID: 15676211 - Flt3 in regulation of type I interferon-producing cell and dendritic cell development.
Onai N, Obata-Onai A, Schmid MA, Manz MG. Onai N, et al. Ann N Y Acad Sci. 2007 Jun;1106:253-61. doi: 10.1196/annals.1392.015. Epub 2007 Mar 14. Ann N Y Acad Sci. 2007. PMID: 17360795 Review. - Adult T-cell progenitors retain myeloid potential.
Wada H, Masuda K, Satoh R, Kakugawa K, Ikawa T, Katsura Y, Kawamoto H. Wada H, et al. Nature. 2008 Apr 10;452(7188):768-72. doi: 10.1038/nature06839. Nature. 2008. PMID: 18401412 - Differentiation of hematopoietic progenitor cells towards the myeloid and B-lymphoid lineage by hepatocyte growth factor (HGF) and thrombopoietin (TPO) together with early acting cytokines.
Grassinger J, Mueller G, Zaiss M, Kunz-Schughart LA, Andreesen R, Hennemann B. Grassinger J, et al. Eur J Haematol. 2006 Aug;77(2):134-44. doi: 10.1111/j.1600-0609.2006.00673.x. Eur J Haematol. 2006. PMID: 16856909 - From plasmacytoid to dendritic cell: morphological and functional switches during plasmacytoid pre-dendritic cell differentiation.
Soumelis V, Liu YJ. Soumelis V, et al. Eur J Immunol. 2006 Sep;36(9):2286-92. doi: 10.1002/eji.200636026. Eur J Immunol. 2006. PMID: 16892183 Review.
Cited by
- Regulation of pDC fate determination by histone deacetylase 3.
Zhang Y, Wu T, He Z, Lai W, Shen X, Lv J, Wang Y, Wu L. Zhang Y, et al. Elife. 2023 Nov 27;12:e80477. doi: 10.7554/eLife.80477. Elife. 2023. PMID: 38011375 Free PMC article. - Plasmacytoid dendritic cells: A dendritic cell in disguise.
Arroyo Hornero R, Idoyaga J. Arroyo Hornero R, et al. Mol Immunol. 2023 Jul;159:38-45. doi: 10.1016/j.molimm.2023.05.007. Epub 2023 Jun 1. Mol Immunol. 2023. PMID: 37269733 Free PMC article. Review. - Clonal Analysis of Human Dendritic Cell Progenitors Using a Stromal Cell Culture.
Liu K, Lee J, Luh T. Liu K, et al. Methods Mol Biol. 2023;2618:155-170. doi: 10.1007/978-1-0716-2938-3_12. Methods Mol Biol. 2023. PMID: 36905516 - Regulation of emergency granulopoiesis during infection.
Paudel S, Ghimire L, Jin L, Jeansonne D, Jeyaseelan S. Paudel S, et al. Front Immunol. 2022 Sep 5;13:961601. doi: 10.3389/fimmu.2022.961601. eCollection 2022. Front Immunol. 2022. PMID: 36148240 Free PMC article. Review. - Unboxing dendritic cells: Tales of multi-faceted biology and function.
Giza HM, Bozzacco L. Giza HM, et al. Immunology. 2021 Nov;164(3):433-449. doi: 10.1111/imm.13394. Epub 2021 Aug 8. Immunology. 2021. PMID: 34309853 Free PMC article. Review.
References
- Kondo, M., A.J. Wagers, M.G. Manz, S.S. Prohaska, D.C. Scherer, G.F. Beilhack, J.A. Shizuru, and I.L. Weissman. 2003. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu. Rev. Immunol. 21:759–806. - PubMed
- Banchereau, J., and R.M. Steinman. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252. - PubMed
- Liu, Y.J. 2001. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell. 106:259–262. - PubMed
- Steinman, R.M., D. Hawiger, and M.C. Nussenzweig. 2003. Tolerogenic dendritic cells. Annu. Rev. Immunol. 21:685–711. - PubMed
- Caux, C., B. Vanbervliet, C. Massacrier, C. Dezutter-Dambuyant, B. de Saint-Vis, C. Jacquet, K. Yoneda, S. Imamura, D. Schmitt, and J. Banchereau. 1996. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+TNFα. J. Exp. Med. 184:695–706. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials