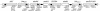Molecular characterization of a beta-lactamase gene, blaGIM-1, encoding a new subclass of metallo-beta-lactamase - PubMed (original) (raw)
Molecular characterization of a beta-lactamase gene, blaGIM-1, encoding a new subclass of metallo-beta-lactamase
Mariana Castanheira et al. Antimicrob Agents Chemother. 2004 Dec.
Abstract
As part of the SENTRY Antimicrobial Surveillance Program in 2002, five multidrug-resistant Pseudomonas aeruginosa clinical isolates were detected with metallo-beta-lactamase (MbetaL) activity. The isolates were recovered from different patients in a medical center located in Dusseldorf, Germany. The resistant determinant was isolated amplifying the region between the integrase and the aacA4 gene cassette. Sequencing revealed a novel MbetaL gene, designated bla(GIM-1). Additional analysis showed that GIM-1, comprising 250 amino acids and with a pI value of 5.4, differs in its primary sequence from that described for IMP, VIM, and SPM-1 enzymes by 39 to 43%, 28 to 31%, and 28%, respectively. The enzyme possesses unique amino acids within the major consensus sequence (HXHXD) of the MbetaL family. Kinetics analysis revealed that GIM-1 has no clear preference for any substrate and did not hydrolyze azlocillin, aztreonam, and the serine-beta-lactamase inhibitors. bla(GIM-1) was found on a 22-kb nontransferable plasmid. The new MbetaL gene was embedded in the first position of a 6-kb class 1 integron, In77, with distinct features, including an aacA4 cassette downstream of the MbetaL gene that appeared to be truncated with bla(GIM-1). The aacA4 was followed by an aadA1 gene cassette that was interrupted by a copy of the IS1394. This integron also carried an oxacillinase gene, bla(OXA-2), before the 3'-CS region. GIM-1 appears to be a unique MbetaL, which is located in a distinct integron structure, and represents the fourth subclass of mobile MbetaL enzymes to be characterized.
Figures
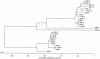
FIG. 1.
Phylogenetic tree obtained for most of the mobile MβLs. The alignment used was prepared with CLUSTALW.
FIG. 2.
Alignment of the amino acid sequence of GIM-1 with three representatives of the MβL groups IMP-1, VIM-1, and SPM-1. Differences in the amino acid sequences are noted by a single letter representing the amino acid change within a particular sequence. Residues involved in the coordination of the zinc iron are underlined and numbered according to the BBL system (in italics).
FIG. 3.
(a) Agarose gel electrophoresis. Lanes: M, 1-kb plus ladder; P, probe; TD, genomic DNA; p5671, plasmid from strain 73-5671. (b) Results of Southern blot analysis showing the hybridization of intl1F/GIMR marked probe with the probe fragment and the 22-kb plasmid from the index isolate harboring _bla_GIM-1.
FIG. 4.
Schematic representation of integron ln77 carrying bla_GIM-1 (the arrows in the gene boxes indicating the direction of transcription). The black dots indicate 59-bes. In the third cassette position the aadA1 is interrupted by a copy of the IS_1394. Block arrows beneath the gene map indicate the positions of the primers used for PCRs and sequence analyses.
Similar articles
- Characterization of acquired beta-lactamases and their genetic support in multidrug-resistant Pseudomonas aeruginosa isolates in Taiwan: the prevalence of unusual integrons.
Yan JJ, Hsueh PR, Lu JJ, Chang FY, Ko WC, Wu JJ. Yan JJ, et al. J Antimicrob Chemother. 2006 Sep;58(3):530-6. doi: 10.1093/jac/dkl266. Epub 2006 Jul 1. J Antimicrob Chemother. 2006. PMID: 16816399 - Integron carrying a novel metallo-beta-lactamase gene, blaIMP-16, and a fused form of aminoglycoside-resistant gene aac(6')-30/aac(6')-Ib': report from the SENTRY Antimicrobial Surveillance Program.
Mendes RE, Toleman MA, Ribeiro J, Sader HS, Jones RN, Walsh TR. Mendes RE, et al. Antimicrob Agents Chemother. 2004 Dec;48(12):4693-702. doi: 10.1128/AAC.48.12.4693-4702.2004. Antimicrob Agents Chemother. 2004. PMID: 15561846 Free PMC article. - Italian metallo-beta-lactamases: a national problem? Report from the SENTRY Antimicrobial Surveillance Programme.
Toleman MA, Biedenbach D, Bennett DM, Jones RN, Walsh TR. Toleman MA, et al. J Antimicrob Chemother. 2005 Jan;55(1):61-70. doi: 10.1093/jac/dkh512. Epub 2004 Dec 1. J Antimicrob Chemother. 2005. PMID: 15574470 - Acquired metallo-β-lactamases and their genetic association with class 1 integrons and ISCR elements in Gram-negative bacteria.
Zhao WH, Hu ZQ. Zhao WH, et al. Future Microbiol. 2015;10(5):873-87. doi: 10.2217/fmb.15.18. Future Microbiol. 2015. PMID: 26000655 Review. - Epidemiology and Characteristics of Metallo-β-Lactamase-Producing Pseudomonas aeruginosa.
Hong DJ, Bae IK, Jang IH, Jeong SH, Kang HK, Lee K. Hong DJ, et al. Infect Chemother. 2015 Jun;47(2):81-97. doi: 10.3947/ic.2015.47.2.81. Epub 2015 Jun 30. Infect Chemother. 2015. PMID: 26157586 Free PMC article. Review.
Cited by
- Structural insights into the subclass B3 metallo-β-lactamase SMB-1 and the mode of inhibition by the common metallo-β-lactamase inhibitor mercaptoacetate.
Wachino J, Yamaguchi Y, Mori S, Kurosaki H, Arakawa Y, Shibayama K. Wachino J, et al. Antimicrob Agents Chemother. 2013 Jan;57(1):101-9. doi: 10.1128/AAC.01264-12. Epub 2012 Oct 15. Antimicrob Agents Chemother. 2013. PMID: 23070156 Free PMC article. - Prevalence of Extended Spectrum β-Lactamase and Antimicrobial Susceptibility Pattern of Clinical Isolates of Pseudomonas from Patients of Khyber Pakhtunkhwa, Pakistan.
Ahmad M, Hassan M, Khalid A, Tariq I, Asad MH, Samad A, Mahmood Q, Murtaza G. Ahmad M, et al. Biomed Res Int. 2016;2016:6068429. doi: 10.1155/2016/6068429. Epub 2016 Jun 5. Biomed Res Int. 2016. PMID: 27366750 Free PMC article. - Genetic and biochemical characterization of a novel metallo-β-lactamase, TMB-1, from an Achromobacter xylosoxidans strain isolated in Tripoli, Libya.
El Salabi A, Borra PS, Toleman MA, Samuelsen Ø, Walsh TR. El Salabi A, et al. Antimicrob Agents Chemother. 2012 May;56(5):2241-5. doi: 10.1128/AAC.05640-11. Epub 2012 Jan 30. Antimicrob Agents Chemother. 2012. PMID: 22290947 Free PMC article. - Metallo-β-lactamases in the Age of Multidrug Resistance: From Structure and Mechanism to Evolution, Dissemination, and Inhibitor Design.
Bahr G, González LJ, Vila AJ. Bahr G, et al. Chem Rev. 2021 Jul 14;121(13):7957-8094. doi: 10.1021/acs.chemrev.1c00138. Epub 2021 Jun 15. Chem Rev. 2021. PMID: 34129337 Free PMC article. Review. - Crystallization and preliminary diffraction studies of GIM-1, a class B carbapenem-hydrolyzing β-lactamase.
Hong MK, Lee JH, Kwon DB, Kim JK, Tran TH, Nguyen DD, Jeong BC, Lee SH, Kang LW. Hong MK, et al. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2012 Oct 1;68(Pt 10):1226-8. doi: 10.1107/S1744309112035695. Epub 2012 Sep 28. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2012. PMID: 23027753 Free PMC article.
References
- Bahar, G., A. Mazzariol, R. Koncan, A. Mert, R. Fontana, G. M. Rossolini, and G. Cornaglia. 2004. Detection of VIM-5 metallo-beta-lactamase in a Pseudomonas aeruginosa clinical isolate from Turkey. J. Antimicrob. Chemother. 54:282-283. - PubMed
- Bennett, P. M. 1999. Integrons and gene cassettes: a genetic construction kit for bacteria. J. Antimicrob. Chemother. - PubMed
- Bush, K. 1998. Metallo-β-lactamases: a class apart. Clin. Infect. Dis. 27(Suppl. 1):S48-S53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous