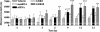Neurochemical and behavioral consequences of widespread gene knockdown in the adult mouse brain by using nonviral RNA interference - PubMed (original) (raw)
Neurochemical and behavioral consequences of widespread gene knockdown in the adult mouse brain by using nonviral RNA interference
Deepak R Thakker et al. Proc Natl Acad Sci U S A. 2004.
Abstract
Gene expression analysis implicates an increasing number of novel genes in the brain as potential targets for the treatment of neurological and psychiatric disorders. Frequently, these genes are ubiquitously expressed in the brain and, thus, may contribute to a pathophysiological state through actions in several brain nuclei. Current strategies employing genetically modified animals for in vivo validation of such targets are time-consuming and often limited by developmental adaptations. Somatic gene manipulation using viral-mediated RNA interference (RNAi) has emerged recently, although restricting the target validation to specific brain nuclei. We investigated whether nonviral infusion of short interfering RNA (siRNA) into the ventricular system would enable a sequence-specific gene knockdown. The temporality and extent of siRNA-induced down-regulation were analyzed by targeting a transgene, EGFP, in mice overexpressing EGFP. Extensive knockdown of EGFP was observed, especially in regions adjacent or dorsoventrally and mediolaterally distant to the infusion site (dorsal third ventricle), with lesser knockdown in more distal regions. We challenged our RNAi approach to generate a specific knockdown of an endogenous gene, encoding the dopamine transporter (DAT) in regions (ventral midbrain) far distal to the infusion site. DAT-siRNA infusion in adult mice produced a significant down-regulation of DAT mRNA and protein in the brain and also elicited a temporal hyperlocomotor response similar to that (but delayed) obtained upon infusion of GBR-12909, a pharmacologically selective DAT inhibitor. Application of this nonviral RNAi approach may accelerate target validation for neuropsychiatric disorders that involve a complex interplay of gene(s) from various brain regions.
Figures
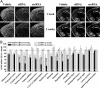
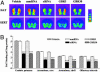
Fig. 1.
siRNA-induced specific, temporal, and widespread knockdown of EGFP mRNA in the brain. EGFP-expressing mice received an infusion of vehicle, EGFP-targeting siRNA, or scrRNA into the dorsal third ventricle for the indicated time. EGFP mRNA levels were reduced, in a temporal fashion, in several brain regions of mice infused with siRNA as compared with levels in vehicle- and scrRNA-treated mice. Levels of GABAAα2 mRNA were unaffected. (A) Low-magnification (×10), dark-field photomicrographs of representatives of in situ hybridization with EGFP (Left) and GABAAα2 (Right) riboprobes in adjacent coronal brain sections, after emulsion-dipping of slides. (B) Densitometric quantification of mRNA-positive grains in each brain region is expressed as percent OD values of those in corresponding regions from vehicle-treated mice. Bars represent means ± SEM of 16–36 observations (four to six observations per animal and four to six animals per group). *, P < 0.05; **, P < 0.01; ***, P < 0.001, significantly different from vehicle- and scrRNA-treated mice in each brain region. #, P < 0.05; ###, P < 0.001, significantly different from siRNA treatment for 1 week in the same region, two-way ANOVA followed by Tukey's post hoc test.
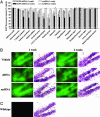
Fig. 2.
siRNA-mediated temporal and extensive down-regulation of EGFP in the mouse brain. (A) Green fluorescence images from microscopic fields of sectioned brain regions were analyzed for quantifying fluorescence intensity as an indicator of EGFP levels in each region. Bars represent mean fluorescence intensity units ± SEM of 20–40 observations (five to eight observations per animal and four to six animals per group). siRNA infusion in the mouse brain time-dependently down-regulated the EGFP levels in several brain regions tested. *, P < 0.05; **, P < 0.01; ***, P < 0.001, significantly different from vehicle- and scrRNA-treated mice in each brain region. #, P < 0.05; ###, P < 0.001, significantly different from siRNA treatment for 1 week in the same region, two-way ANOVA followed by Tukey's post hoc test. (B) Representative microscopic fluorescence images of an area of the dentate gyrus demonstrating a temporal reduction of EGFP levels in siRNA-treated mice as compared with those in vehicle- or scrRNA-treated mice. Nissl-stained adjacent brain sections also are shown for each treatment. (C) Representative microscopic fluorescence image indicating lack of any fluorescence emitted from the same area of dentate gyrus of wild-type mice that do not express EGFP. Nissl staining of adjacent section is shown. (Scale bar, 50 μm.)
Fig. 3.
Effects of infusing a DAT-targeting siRNA or the pharmacological inhibitor GBR-12909 on mouse locomotor activity. Locomotor activity was assessed for 30 min on the indicated days, 0 being the day of surgical implantation of the minipump-cannula assembly for infusing vehicle, DAT-targeting siRNA, mmRNA, or GBR-12909 (GBR3, 3 μg/day; GBR30, 30 μg/day) in the dorsal third ventricle of adult mice. Bars represent mean distance traveled (cm) ± SEM from six animals per group. Infusion of GBR-12909 (30 μg/day) resulted in a significant hyperlocomotor activity in mice on all test days. siRNA elicited a trend toward a significant hyperlocomotion effect on day 9 that was significant on days 12 and 14. *, P < 0.05; **, P < 0.01; ***, P < 0.001, significantly different from vehicle, using repeated-measure ANOVA with treatment as the between factor and time as the within-subject variable, followed by Fisher's post hoc test.
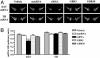
Fig. 4.
siRNA-induced specific knockdown of DAT mRNA in the substantia nigra and ventral tegmental area. Mice received a 2-week infusion of vehicle, DAT-targeting siRNA, mmRNA, or GBR-12909 (3 or 30 μg/day) into the dorsal third ventricle. DAT mRNA levels were reduced significantly in the ventral midbrain regions of mice infused with siRNA. Levels of TH mRNA in the same regions were unaffected by any treatment. (A) Low-magnification (×20), dark-field photomicrographs of representatives of in situ hybridization with DAT (Upper) and TH (Lower) riboprobes in adjacent brain sections, after emulsion-dipping of slides. (B) Densitometric quantification of mRNA positive grains is presented as mean OD values ± SEM of 20–24 observations (two to four observations per animal and six animals per group). ***, P < 0.001, significantly different from all other treatments, using one-way ANOVA followed by Tukey's post hoc test.
Fig. 5.
Down-regulation of the DAT protein after infusion of siRNA or GBR-12909. (A) Representative autoradiograms of [125I]RTI-55 binding to DAT (Upper) or SERT (Lower) in adjacent brain sections of mice infused for 2 weeks with vehicle, DAT-targeting siRNA, mmRNA, or GBR-12909 (3 or 30 μg/day). siRNA or GBR-12909 (30 μg/day) produced a significant down-regulation of DAT in all of the dopaminergic projection areas of the forebrain. None of the treatments produced a significant alteration of SERT protein levels in the same brain regions. (Microscale bars, from top to bottom, represent binding values of 1.52, 2.58, 5.16, and 10.96 nCi/mg.) (B) Quantification of DAT binding in the caudate putamen, accumbens core and shell, and olfactory tubercles. Bars represent mean nCi/mg tissue protein ± SEM of 36–40 observations (six to eight observations per animal and six animals per group). ***, P < 0.001, significantly different from all other treatments, using one-way ANOVA followed by Tukey's post hoc test.
Similar articles
- Local knockdown of genes in the brain using small interfering RNA: a phenotypic comparison with knockout animals.
Salahpour A, Medvedev IO, Beaulieu JM, Gainetdinov RR, Caron MG. Salahpour A, et al. Biol Psychiatry. 2007 Jan 1;61(1):65-9. doi: 10.1016/j.biopsych.2006.03.020. Epub 2006 May 19. Biol Psychiatry. 2007. PMID: 16712807 - Global down-regulation of gene expression in the brain using RNA interference, with emphasis on monoamine transporters and GPCRs: implications for target characterization in psychiatric and neurological disorders.
Hoyer D, Thakker DR, Natt F, Maier R, Huesken D, Müller M, Flor P, VAN DER Putten H, Schmutz M, Bilbe G, Cryan JF. Hoyer D, et al. J Recept Signal Transduct Res. 2006;26(5-6):527-47. doi: 10.1080/10799890600929663. J Recept Signal Transduct Res. 2006. PMID: 17118797 - Emerging use of non-viral RNA interference in the brain.
Cryan JF, Thakker DR, Hoyer D. Cryan JF, et al. Biochem Soc Trans. 2007 Apr;35(Pt 2):411-5. doi: 10.1042/BST0350411. Biochem Soc Trans. 2007. PMID: 17371288 - Interfering with the brain: use of RNA interference for understanding the pathophysiology of psychiatric and neurological disorders.
Thakker DR, Hoyer D, Cryan JF. Thakker DR, et al. Pharmacol Ther. 2006 Mar;109(3):413-38. doi: 10.1016/j.pharmthera.2005.08.006. Epub 2005 Sep 23. Pharmacol Ther. 2006. PMID: 16183135 Review. - Nonviral vector-mediated RNA interference: its gene silencing characteristics and important factors to achieve RNAi-based gene therapy.
Takahashi Y, Nishikawa M, Takakura Y. Takahashi Y, et al. Adv Drug Deliv Rev. 2009 Jul 25;61(9):760-6. doi: 10.1016/j.addr.2009.04.006. Epub 2009 Apr 20. Adv Drug Deliv Rev. 2009. PMID: 19386274 Review.
Cited by
- NLRP1 inflammasome is activated in patients with medial temporal lobe epilepsy and contributes to neuronal pyroptosis in amygdala kindling-induced rat model.
Tan CC, Zhang JG, Tan MS, Chen H, Meng DW, Jiang T, Meng XF, Li Y, Sun Z, Li MM, Yu JT, Tan L. Tan CC, et al. J Neuroinflammation. 2015 Jan 28;12:18. doi: 10.1186/s12974-014-0233-0. J Neuroinflammation. 2015. PMID: 25626361 Free PMC article. - Ultrasound enhanced delivery of molecular imaging and therapeutic agents in Alzheimer's disease mouse models.
Raymond SB, Treat LH, Dewey JD, McDannold NJ, Hynynen K, Bacskai BJ. Raymond SB, et al. PLoS One. 2008 May 14;3(5):e2175. doi: 10.1371/journal.pone.0002175. PLoS One. 2008. PMID: 18478109 Free PMC article. - Simultaneous silencing of Npy and Dbh expression in hindbrain A1/C1 catecholamine cells suppresses glucoprivic feeding.
Li AJ, Wang Q, Dinh TT, Ritter S. Li AJ, et al. J Neurosci. 2009 Jan 7;29(1):280-7. doi: 10.1523/JNEUROSCI.4267-08.2009. J Neurosci. 2009. PMID: 19129404 Free PMC article. - Involvement of long noncoding RNAs in diseases affecting the central nervous system.
Pastori C, Wahlestedt C. Pastori C, et al. RNA Biol. 2012 Jun;9(6):860-70. doi: 10.4161/rna.20482. Epub 2012 Jun 1. RNA Biol. 2012. PMID: 22699553 Free PMC article. Review. - Acute Neurotoxicity of Antisense Oligonucleotides After Intracerebroventricular Injection Into Mouse Brain Can Be Predicted from Sequence Features.
Hagedorn PH, Brown JM, Easton A, Pierdomenico M, Jones K, Olson RE, Mercer SE, Li D, Loy J, Høg AM, Jensen ML, Gill M, Cacace AM. Hagedorn PH, et al. Nucleic Acid Ther. 2022 Jun;32(3):151-162. doi: 10.1089/nat.2021.0071. Epub 2022 Feb 14. Nucleic Acid Ther. 2022. PMID: 35166597 Free PMC article.
References
- Mirnics, K., Middleton, F. A., Marques, A., Lewis, D. & Levitt, P. (2000) Neuron 28, 53–67. - PubMed
- McClung, C. A. & Nestler, E. J. (2003) Nat. Neurosci. 6, 1208–1215. - PubMed
- Dorsett, Y. & Tuschl, T. (2004) Nat. Rev. Drug Discov. 3, 318–329. - PubMed
- Fire, A., Xu, S., Montgomery, M. K., Kostas, S. A., Driver, S. E. & Mello, C. C. (1998) Nature 391, 806–811. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources