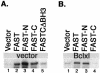FAST is a survival protein that senses mitochondrial stress and modulates TIA-1-regulated changes in protein expression - PubMed (original) (raw)
FAST is a survival protein that senses mitochondrial stress and modulates TIA-1-regulated changes in protein expression
Wei Li et al. Mol Cell Biol. 2004 Dec.
Abstract
The Fas-activated serine/threonine phosphoprotein (FAST) is tethered to the outer mitochondrial membrane, where it interacts with BCL-X(L) (17). Here we show that RNA interference-mediated knockdown of endogenous FAST results in apoptosis, whereas overexpressed recombinant FAST inhibits Fas- and UV-induced apoptosis, indicating that FAST is a survival protein. The antiapoptotic effects of FAST are regulated by interactions with the translational silencer TIA-1: a FAST mutant lacking its TIA-1-binding domain does not inhibit apoptosis, and overexpressed recombinant TIA-1 inhibits the antiapoptotic effects of FAST. Because the antiapoptotic effects of FAST require ongoing protein synthesis, we hypothesized that FAST might function by preventing TIA-1-mediated silencing of mRNAs encoding inhibitors of apoptosis. Consistent with this hypothesis, FAST promotes the expression of cotransfected reporter proteins, a process that requires its TIA-1-binding domain and is inhibited by overexpressed recombinant TIA-1. More compellingly, recombinant FAST increases the expression of endogenous cIAP-1 and XIAP, but not GAPDH, in transfected HeLa cells. Because FAST is released from mitochondria in cells undergoing Fas- or UV-induced apoptosis, we propose that FAST serves as a sensor of mitochondrial stress that modulates a TIA-1-regulated posttranscriptional stress response program.
Figures
FIG. 1.
Knocking down FAST results in apoptosis. (A) COS-7 cells were transfected with pMT2-HA-FAST together with a vector-based negative control RNAi (pSuppressor2-SiNC), FAST RNAi-1 (pSuppressor2-SiFAST-1), or FAST RNAi-2 (pSupressor2-SiFAST-2). After 48 h, cells were processed for Western blotting analysis to quantify the expression of recombinant HA-FAST. (B) HeLa cells were transfected with either negative control RNAi (SiNC) or FAST RNAi (SiFAST-1). After 48 h, cells were processed for Western blotting analysis to quantify the expression of endogenous FAST. (C) HeLa cells were transfected with pcDNA3-β-galactosidase together with either negative control RNAi (SiNC) or FAST RNAi (siFAST-1 or siFAST-2) before being processed for immunofluorescence microscopy using anti-β-galactosidase, anti-active caspase 3, or Hoechst dye. Arrows point out healthy transfected cells. Arrowheads point out transfected cells that are undergoing apoptosis. Size bar, 20 μm. (D) The mean percentages (±standard errors; n = 3) of transfected cells (revealed using anti-β-galactosidase) that exhibit active caspase-3 are presented as a bar graph. Calculated P values for selected comparisons are shown. β-gal, β-galactosidase.
FIG. 1.
Knocking down FAST results in apoptosis. (A) COS-7 cells were transfected with pMT2-HA-FAST together with a vector-based negative control RNAi (pSuppressor2-SiNC), FAST RNAi-1 (pSuppressor2-SiFAST-1), or FAST RNAi-2 (pSupressor2-SiFAST-2). After 48 h, cells were processed for Western blotting analysis to quantify the expression of recombinant HA-FAST. (B) HeLa cells were transfected with either negative control RNAi (SiNC) or FAST RNAi (SiFAST-1). After 48 h, cells were processed for Western blotting analysis to quantify the expression of endogenous FAST. (C) HeLa cells were transfected with pcDNA3-β-galactosidase together with either negative control RNAi (SiNC) or FAST RNAi (siFAST-1 or siFAST-2) before being processed for immunofluorescence microscopy using anti-β-galactosidase, anti-active caspase 3, or Hoechst dye. Arrows point out healthy transfected cells. Arrowheads point out transfected cells that are undergoing apoptosis. Size bar, 20 μm. (D) The mean percentages (±standard errors; n = 3) of transfected cells (revealed using anti-β-galactosidase) that exhibit active caspase-3 are presented as a bar graph. Calculated P values for selected comparisons are shown. β-gal, β-galactosidase.
FIG. 2.
FAST inhibits Fas- and UV-induced apoptosis. (A) HeLa cells were mock transfected or transfected with vectors encoding HA-FAST or β-galactosidase, cultured in the absence (untreated) or presence of anti-Fas antibody (FAS) before being processed for immunofluorescence microscopy using anti-HA, anti-β-galactosidase, anti-active caspase 3, and Hoechst dye. Arrows point out cells undergoing Fas-induced caspase-dependent apoptosis. Arrowheads point out FAST transfectants in which caspase 3 is not activated. Size bar, 20 μm. (B) HeLa cells were mock transfected or transfected with either FAST or β-galactosidase (β-gal), cultured in the presence of anti-Fas antibody, and then processed for TUNEL analysis (red) and immunofluorescence (anti-HA, green; anti-active caspase 3, blue) or Hoechst staining. Arrows point out untransfected cells or β-galactosidase-transfected cells. Arrowheads point out FAST transfectants. (C) The mean percentages (±standard errors; n = 3) of transfected cells (vector control, HA-FAST, HA-FASTN, or HA-FASTC; revealed using anti-HA or anti-β-galactosidase) cultured under the indicated conditions that exhibit active caspase 3 are presented as a bar graph. Calculated P values for selected comparisons are shown.
FIG. 2.
FAST inhibits Fas- and UV-induced apoptosis. (A) HeLa cells were mock transfected or transfected with vectors encoding HA-FAST or β-galactosidase, cultured in the absence (untreated) or presence of anti-Fas antibody (FAS) before being processed for immunofluorescence microscopy using anti-HA, anti-β-galactosidase, anti-active caspase 3, and Hoechst dye. Arrows point out cells undergoing Fas-induced caspase-dependent apoptosis. Arrowheads point out FAST transfectants in which caspase 3 is not activated. Size bar, 20 μm. (B) HeLa cells were mock transfected or transfected with either FAST or β-galactosidase (β-gal), cultured in the presence of anti-Fas antibody, and then processed for TUNEL analysis (red) and immunofluorescence (anti-HA, green; anti-active caspase 3, blue) or Hoechst staining. Arrows point out untransfected cells or β-galactosidase-transfected cells. Arrowheads point out FAST transfectants. (C) The mean percentages (±standard errors; n = 3) of transfected cells (vector control, HA-FAST, HA-FASTN, or HA-FASTC; revealed using anti-HA or anti-β-galactosidase) cultured under the indicated conditions that exhibit active caspase 3 are presented as a bar graph. Calculated P values for selected comparisons are shown.
FIG. 3.
(A) Stress stimuli displace endogenous FAST from mitochondria. HeLa cells were left untreated or were treated with UV irradiation (10 mJ/cm2 followed by 0.5 or 1 h recovery) or anti-Fas antibody (1:200 dilution from culture supernatants) for 6 h before being processed to obtain pellets enriched in mitochondria (P20) and corresponding supernatants (S20). Individual fractions were processed for Western blotting analysis with anti-FASTN antibody or anti-BCL-XL antibody. (B) Fas ligation interrupts FAST/BCL-XL interaction. HeLa cell extracts prepared from cells cultured in the absence or presence of anti-Fas antibody were processed for coimmunoprecipitation with either rabbit anti-BCL-XL antibody or an isotype-matched control antibody (rabbit anti-Myc). Immunoprecipitates were then processed for Western blotting analysis with anti-FAST-N antibody or mouse anti-BCL-XL antibody.
FIG. 4.
Identification of FAST/TIA1 interaction sites. (A) Schematic depiction of HA-FASTN and HA-FASTC truncation mutants. MTD, mitochondrial tethering domain; BH3, BCL2 homology 3-related domain. (B) Schematic depiction of HA-TIA-1 truncation mutants. 1, 2, 3, RNA-recognition motifs; PRD, prion-related domain. (C) COS-7 cells were cotransfected with pcDNA3-FLAG-TIA1 and the indicated HA-FAST truncation mutants. After 28 h, cells were processed for coimmunoprecipitation analysis with mouse anti-HA antibody followed by Western blotting analysis with mouse anti-FLAG antibody, followed by mouse anti-HA antibody. Lysates from transfected cells were analyzed by Western blotting with anti-FLAG antibody (load). (D) COS-7 cells were cotransfected with pcDNA3-FLAG-FAST and the indicated HA-TIA1 truncation mutants. Lysates from transfected cells were immunoprecipitated with anti-HA antibody and then were sequentially blotted with anti-FLAG antibody and anti-HA antibody. Lysates from transfected cells were analyzed by Western blotting with anti-FLAG antibody (load). FL, FLAG.
FIG. 5.
TIA-1 inhibits the antiapoptotic effects of FAST. HeLa cells were transfected with the indicated constructs, cultured in the absence (untreated) or presence (Fas) of anti-Fas antibody, and then processed for immunofluorescence microscopy using anti-HA (or anti-β-galactosidase), anti-active caspase 3, or Hoechst dye. The mean percentages (±standard errors; n = 3) of transfected cells (revealed using anti-HA or anti-β-galactosidase) cultured under the indicated conditions that exhibit active caspase 3 are presented as bar graphs. Calculated P values for selected comparisons are shown.
FIG. 6.
FAST enhances the expression of cotransfected β-galactosidase. The left panel shows COS-7 cells cotransfected with a β-galactosidase reporter together with pMT2 alone, pMT2-HA-FAST, pMT2-HA-TIA-1, or pMT2-HA-TIA-1-PRD. After 48 h, cells were processed for Western blotting analysis to quantify the expression of β-galactosidase (β-gal). The right panel shows COS-7 cells cotransfected with a β-galactosidase reporter together with pMT2-HA-FAST and increasing amounts of pMT2-HA-TIA-1 (5:1, 2:1, 1:1). After 48 h, cells were processed for Western blotting analysis to quantify the expression of β-galactosidase and the expression of recombinant TIA-1 and FAST.
FIG. 7.
FASTC increases reporter gene expression and is regulated by BCL-XL. (A) COS-7 cells were cotransfected with a β-galactosidase reporter together with pcDNA3 vector and pMT2 alone, pMT2-HA-FAST, pMT2-HA-FASTN, pMT2-HA-FASTC, or pMT2-HA-FASTCΔBH3. After 48 h, cells were processed for Western blotting analysis to quantify the expression of β-galactosidase. (B) COS-7 cells were cotransfected with a β-galactosidase reporter together with pcDNA3-BCL-XL and pMT2 alone, pMT2-HA-FAST, pMT2-HA-FASTN, or pMT2-HA-FASTC.
FIG. 8.
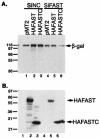
Effects of siFAST-1 on β-galactosidase expression. COS-7 cells were cotransfected with a β-galactosidase (β-gal) reporter together with pMT2-HA-FAST, pMT2-HA-FASTN, or pMT2-HA-FASTC and either pSupressor2-siNC (lanes 1 to 3) or pSupressor-2-siFAST-1 (lanes 4 to 6). After 48 h, cells were processed for Western blotting analysis to quantify the expression of β-galactosidase (A) or individual HA-FAST constructs (B). Molecular size markers are shown at the left. Bands corresponding to β-galactosidase, HA-FAST, and HA-FASTC are indicated by arrows.
FIG. 9.
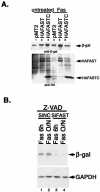
Fas ligation modulates the expression of β-galactosidase. (A) HeLa cells were cotransfected with a β-galactosidase reporter together with pMT2 vector, pMT2-HA-FAST, or pMT2-HA-FASTC, and then they were cultured in the absence or presence of anti-Fas antibody before being processed for Western blotting analysis to quantify the expression of β-galactosidase (β-gal) (upper panel), HA-FAST, and HA-FASTC (lower panel). (B) HeLa cells were transfected with negative control siRNA or siFAST-1 and cultured for 28 or 36 h in the presence of z-VAD (100 nM; Enzyme Systems Products), and then they were treated with anti-Fas antibody for 6 h or overnight, as indicated, before being processed for immunoblotting analysis to quantify the expression of β-galactosidase and endogenous GAPDH.
FIG. 10.
The antiapoptotic effects of FAST require active NF-κB and ongoing protein synthesis. HeLa cells were transfected with the indicated constructs and then were cultured in the absence (untreated) or presence of anti-Fas antibody (Fas), with or without cycloheximide (CHX; 0.025 μg/ml), before being processed for immunofluorescence microscopy using anti-HA (or anti-β-galactosidase), anti-caspase 3, or Hoechst dye. The mean percentages (±standard errors; n = 3) of transfected cells (revealed using anti-HA or anti-β-galactosidase) that exhibit active caspase-3 are presented as bar graphs. Calculated P values for selected comparisons are shown.
FIG. 11.
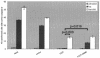
FAST promotes the expression of cIAP-1 and XIAP in HeLa transfectants. (A) HeLa cells were transfected with cDNAs encoding a vector control or recombinant FAST. After 48 h, cells were processed for Western blotting to quantify the expression of endogenous cIAP-1, XIAP, and GAPDH. These blots are representative of three independent experiments. (B) The expression of cIAP-1 and XIAP was quantified by densitometry. Protein expression in vector transfectants was assigned a value of 1. The relative increase in expression observed in FAST transfectants is indicated as means ± standard errors (n = 3). P values were calculated using the Student's t test.
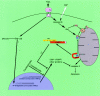
FIG. 12.
Proposed mechanism by which FAST inhibits Fas-induced apoptosis.
Similar articles
- FAST is a BCL-X(L)-associated mitochondrial protein.
Li W, Kedersha N, Chen S, Gilks N, Lee G, Anderson P. Li W, et al. Biochem Biophys Res Commun. 2004 May 21;318(1):95-102. doi: 10.1016/j.bbrc.2004.03.188. Biochem Biophys Res Commun. 2004. PMID: 15110758 - Fas-activated serine/threonine kinase (FAST K) synergizes with TIA-1/TIAR proteins to regulate Fas alternative splicing.
Izquierdo JM, Valcárcel J. Izquierdo JM, et al. J Biol Chem. 2007 Jan 19;282(3):1539-43. doi: 10.1074/jbc.C600198200. Epub 2006 Nov 29. J Biol Chem. 2007. PMID: 17135269 - Fas-activated serine/threonine kinase (FAST) phosphorylates TIA-1 during Fas-mediated apoptosis.
Tian Q, Taupin J, Elledge S, Robertson M, Anderson P. Tian Q, et al. J Exp Med. 1995 Sep 1;182(3):865-74. doi: 10.1084/jem.182.3.865. J Exp Med. 1995. PMID: 7544399 Free PMC article. - Upstream regulatory role for XIAP in receptor-mediated apoptosis.
Wilkinson JC, Cepero E, Boise LH, Duckett CS. Wilkinson JC, et al. Mol Cell Biol. 2004 Aug;24(16):7003-14. doi: 10.1128/MCB.24.16.7003-7014.2004. Mol Cell Biol. 2004. PMID: 15282301 Free PMC article. - The FASTK family of proteins: emerging regulators of mitochondrial RNA biology.
Jourdain AA, Popow J, de la Fuente MA, Martinou JC, Anderson P, Simarro M. Jourdain AA, et al. Nucleic Acids Res. 2017 Nov 2;45(19):10941-10947. doi: 10.1093/nar/gkx772. Nucleic Acids Res. 2017. PMID: 29036396 Free PMC article. Review.
Cited by
- Mammalian stress granules and P bodies at a glance.
Riggs CL, Kedersha N, Ivanov P, Anderson P. Riggs CL, et al. J Cell Sci. 2020 Sep 1;133(16):jcs242487. doi: 10.1242/jcs.242487. J Cell Sci. 2020. PMID: 32873715 Free PMC article. Review. - Fast kinase domain-containing protein 3 is a mitochondrial protein essential for cellular respiration.
Simarro M, Gimenez-Cassina A, Kedersha N, Lazaro JB, Adelmant GO, Marto JA, Rhee K, Tisdale S, Danial N, Benarafa C, Orduña A, Anderson P. Simarro M, et al. Biochem Biophys Res Commun. 2010 Oct 22;401(3):440-6. doi: 10.1016/j.bbrc.2010.09.075. Epub 2010 Sep 24. Biochem Biophys Res Commun. 2010. PMID: 20869947 Free PMC article. - Stress granules and processing bodies are dynamically linked sites of mRNP remodeling.
Kedersha N, Stoecklin G, Ayodele M, Yacono P, Lykke-Andersen J, Fritzler MJ, Scheuner D, Kaufman RJ, Golan DE, Anderson P. Kedersha N, et al. J Cell Biol. 2005 Jun 20;169(6):871-84. doi: 10.1083/jcb.200502088. J Cell Biol. 2005. PMID: 15967811 Free PMC article. - miR-106a-5p inhibits the proliferation and migration of astrocytoma cells and promotes apoptosis by targeting FASTK.
Zhi F, Zhou G, Shao N, Xia X, Shi Y, Wang Q, Zhang Y, Wang R, Xue L, Wang S, Wu S, Peng Y, Yang Y. Zhi F, et al. PLoS One. 2013 Aug 27;8(8):e72390. doi: 10.1371/journal.pone.0072390. eCollection 2013. PLoS One. 2013. PMID: 24013584 Free PMC article. - FASTKD2 and human memory: functional pathways and prospects for novel therapeutic target development for Alzheimer's disease and age-associated memory decline.
Ramanan VK, Saykin AJ. Ramanan VK, et al. Pharmacogenomics. 2015;16(5):429-32. doi: 10.2217/pgs.15.8. Pharmacogenomics. 2015. PMID: 25916514 Free PMC article. No abstract available.
References
- Anderson, P., and N. Kedersha. 2002. Stressful initiations. J. Cell Sci. 115:3227-3234. - PubMed
- Barber, G. N. 2001. Host defense, viruses and apoptosis. Cell Death Differ. 8:113-126. - PubMed
- Barnhart, B., E. Alappat, and M. E. Peter. 2003. The CD95 Thpe I/type II model. Semin. Immunol. 15:185-193. - PubMed
- Cheng, E. H., M. C. Wei, S. Weiler, R. A. Flavell, T. W. Mak, T. Lindsten, and S. J. Korsmeyer. 2001. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol. Cell 8:705-711. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AR 051472/AR/NIAMS NIH HHS/United States
- R56 AI033600/AI/NIAID NIH HHS/United States
- R01 AI033600/AI/NIAID NIH HHS/United States
- AI 50167/AI/NIAID NIH HHS/United States
- R01 AI050167/AI/NIAID NIH HHS/United States
- R01 AR051472/AR/NIAMS NIH HHS/United States
- AI 33600/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous