Radial glia give rise to adult neural stem cells in the subventricular zone - PubMed (original) (raw)
Radial glia give rise to adult neural stem cells in the subventricular zone
Florian T Merkle et al. Proc Natl Acad Sci U S A. 2004.
Abstract
Neural stem cells with the characteristics of astrocytes persist in the subventricular zone (SVZ) of the juvenile and adult brain. These cells generate large numbers of new neurons that migrate through the rostral migratory stream to the olfactory bulb. The developmental origin of adult neural stem cells is not known. Here, we describe a lox-Cre-based technique to specifically and permanently label a restricted population of striatal radial glia in newborn mice. Within the first few days after labeling, these radial glial cells gave rise to neurons, oligodendrocytes, and astrocytes, including astrocytes in the SVZ. Remarkably, the rostral migratory stream contained labeled migratory neuroblasts at all ages examined, including 150-day-old mice. Labeling dividing cells with the S-phase marker BrdUrd showed that new neurons continue to be produced in the adult by precursors ultimately derived from radial glia. Furthermore, both radial glia in neonates and radial glia-derived cells in the adult lateral ventricular wall generated self-renewing, multipotent neurospheres. These results demonstrate that radial glial cells not only serve as progenitors for many neurons and glial cells soon after birth but also give rise to adult SVZ stem cells that continue to produce neurons throughout adult life. This study identifies and provides a method to genetically modify the lineage that links neonatal and adult neural stem cells.
Figures
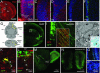
Fig. 1.
Radial glia are labeled specifically in the neonatal mouse brain. (A) RC2+ radial glial processes extend from the VZ to the brain surface. (B_–_F) In the region boxed in A, the disappearance of RC2+ radial glia correlates with the appearance of GFAP+ SVZ astrocytes. (G) Radial glial processes can be infected by an Ad injected into the ventrolateral striatum. (H) This injection results in the labeling of cells at the injection site and in the dorsolateral VZ. (I) VZ cells (box in H) have long radial processes that express RC2 (boxed region) and extend from the VZ through the injection site. (J) These cells have the ultrastructure of radial glia. (J Inset) In the same cells (from P2 R26R mice), X-Gal precipitate (arrowheads) is shown at the light microscope. (K and L) In the early postnatal brain, radial glia retract their processes (arrowheads) as they transform into astrocytes in the striatum (K) and in the SVZ (L). (M_–_P) Their progeny, however, continue to generate large numbers of neuroblasts in the P90 brain (M) that migrate along the RMS to the olfactory bulb (N) and differentiate into granular (O) and periglomerular (P) cells. Cb, cerebellum; Ctx, cortex; LV, lateral ventricle; Stri, striatum.
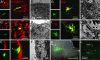
Fig. 2.
Radial glia produce all classes of brain cells. (A_–_J) Radial glial progeny were identified with the confocal microscope by EGFP expression in Z/EG mice and with the electron microscope by dark, perinuclear grains of X-Gal precipitate in R26R mice (black arrowheads). The progeny include doublecortin+ neuroblasts (type A cells) (A and B), NeuN+ neurons (C), ciliated (arrows) CD24+ ependymal cells (E and F), and Olig2+ oligodendrocytes whose processes (arrows) intercalate between myelinated axons (G and H). (D, I, and J) Radial glia also produce GFAP+ SVZ astrocytes or type B cells (I and J; intermediate filaments shown in Inset) and neurogenic transit-amplifying type C cells (D). (K) A whole mount of the lateral ventricular wall reveals the injection site deep in the tissue at a deeper focal plane (yellow arrowheads) and a well defined patch of labeled radial glial cell bodies (arrow). (L) When grafted into a wild-type mouse, radial glial cell bodies (but not cell bodies from the injection site) produce all cell types obtained by viral injection alone, including neuroblasts (arrow) and olfactory bulb neurons (yellow arrowhead) in the adult brain. GCL, granule cell layer.
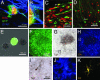
Fig. 3.
Radial glia-derived adult stem cells remain actively neurogenic in vivo. (A) Radial glia-derived (EGFP+) GFAP+ astrocytes in the adult (P33) SVZ are labeled by the S-phase marker BrdUrd. (B) GFAP EGFP double-labeling shown at higher magnification. (C) We also found many double-labeled migratory neuroblasts. (D) These adult-born neuroblasts migrate to the olfactory bulb and differentiate into interneurons. Radial glia and their adult progeny are self-renewing and multipotent in vitro. (E) EGFP+ cells isolated from the ventricular wall of P2, P10, and P90 Z/EG mice injected with Ad:Cre in the ventrolateral striatum at P0 clonally produce primary neurospheres. These EGFP+ neurospheres were sorted by FACS and grown again at clonal density for several passages to ensure clonality and self-renewal. (F_–_H) All cells in clonally grown neurospheres are EGFP+ (F) and differentiate into Tuj1+ neurons (visualized by diaminobenzidine, dark brown spots in G), GFAP+ astrocytes, and O4+ oligodendrocytes (H). (I) A higher magnification of a Tuj1+ neuron (box in G). (J and K) GFAP+ processes of astrocytes (J) and an O4+ oligodendrocyte (K) (box in H). The differentiated neurosphere shown in F_–_K was isolated from a P90 mouse, but we obtained similar results from all ages we examined, demonstrating that both radial glia and their progeny are multipotent, self-renewing stem cells. (F_–_H and I_–_K share the same scale bar.)
Similar articles
- The heterogeneity of adult neural stem cells and the emerging complexity of their niche.
Alvarez-Buylla A, Kohwi M, Nguyen TM, Merkle FT. Alvarez-Buylla A, et al. Cold Spring Harb Symp Quant Biol. 2008;73:357-65. doi: 10.1101/sqb.2008.73.019. Epub 2008 Nov 6. Cold Spring Harb Symp Quant Biol. 2008. PMID: 19022766 Review. - Multiple cell populations in the early postnatal subventricular zone take distinct migratory pathways: a dynamic study of glial and neuronal progenitor migration.
Suzuki SO, Goldman JE. Suzuki SO, et al. J Neurosci. 2003 May 15;23(10):4240-50. doi: 10.1523/JNEUROSCI.23-10-04240.2003. J Neurosci. 2003. PMID: 12764112 Free PMC article. - Lineage analysis of quiescent regenerative stem cells in the adult brain by genetic labelling reveals spatially restricted neurogenic niches in the olfactory bulb.
Giachino C, Taylor V. Giachino C, et al. Eur J Neurosci. 2009 Jul;30(1):9-24. doi: 10.1111/j.1460-9568.2009.06798.x. Epub 2009 Jun 25. Eur J Neurosci. 2009. PMID: 19558606 - Multipotent neural stem cells reside into the rostral extension and olfactory bulb of adult rodents.
Gritti A, Bonfanti L, Doetsch F, Caille I, Alvarez-Buylla A, Lim DA, Galli R, Verdugo JM, Herrera DG, Vescovi AL. Gritti A, et al. J Neurosci. 2002 Jan 15;22(2):437-45. doi: 10.1523/JNEUROSCI.22-02-00437.2002. J Neurosci. 2002. PMID: 11784788 Free PMC article. - Radial glial origin of the adult neural stem cells in the subventricular zone.
Bonfanti L, Peretto P. Bonfanti L, et al. Prog Neurobiol. 2007 Sep;83(1):24-36. doi: 10.1016/j.pneurobio.2006.11.002. Epub 2006 Dec 29. Prog Neurobiol. 2007. PMID: 17196731 Review.
Cited by
- Aberrations of Genomic Imprinting in Glioblastoma Formation.
Lozano-Ureña A, Jiménez-Villalba E, Pinedo-Serrano A, Jordán-Pla A, Kirstein M, Ferrón SR. Lozano-Ureña A, et al. Front Oncol. 2021 Mar 12;11:630482. doi: 10.3389/fonc.2021.630482. eCollection 2021. Front Oncol. 2021. PMID: 33777782 Free PMC article. Review. - Platelet-derived growth factor C deficiency in C57BL/6 mice leads to abnormal cerebral vascularization, loss of neuroependymal integrity, and ventricular abnormalities.
Fredriksson L, Nilsson I, Su EJ, Andrae J, Ding H, Betsholtz C, Eriksson U, Lawrence DA. Fredriksson L, et al. Am J Pathol. 2012 Mar;180(3):1136-1144. doi: 10.1016/j.ajpath.2011.12.006. Epub 2012 Jan 9. Am J Pathol. 2012. PMID: 22230248 Free PMC article. - Orchestrating transcriptional control of adult neurogenesis.
Hsieh J. Hsieh J. Genes Dev. 2012 May 15;26(10):1010-21. doi: 10.1101/gad.187336.112. Genes Dev. 2012. PMID: 22588716 Free PMC article. Review. - A Role for the Cannabinoid 1 Receptor in Neuronal Differentiation of Adult Spinal Cord Progenitors in vitro is Revealed through Pharmacological Inhibition and Genetic Deletion.
Sideris A, Bekker T, Chan WS, Montoya-Gacharna JV, Blanck TJ, Recio-Pinto E. Sideris A, et al. Front Neurosci. 2012 Jan 24;6:4. doi: 10.3389/fnins.2012.00004. eCollection 2012. Front Neurosci. 2012. PMID: 22291615 Free PMC article. - Creation and validation of 3D-printed head molds for stereotaxic injections of neonatal mouse brains.
Chervonski E, Brockman AA, Khurana R, Chen Y, Greenberg S, Hay MS, Luo Y, Miller J, Patelis D, Whitney SK, Walker M 3rd, Ihrie RA. Chervonski E, et al. J Neurosci Methods. 2021 Aug 1;360:109255. doi: 10.1016/j.jneumeth.2021.109255. Epub 2021 Jun 15. J Neurosci Methods. 2021. PMID: 34139267 Free PMC article.
References
- Morshead, C. M., Reynolds, B. A., Craig, C. G., McBurney, M. W., Staines, W. A., Morassutti, D., Weiss, S. & van der Kooy, D. (1994) Neuron 13, 1071–1082. - PubMed
- Gage, F. H. (2000) Science 287, 1433–1438. - PubMed
- Temple, S. (2001) Nature 414, 112–117. - PubMed
- Luskin, M. B. (1998) J. Neurobiol. 36, 221–233. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical