Nano-to-micro scale dynamics of P-selectin detachment from leukocyte interfaces. III. Numerical simulation of tethering under flow - PubMed (original) (raw)
Comparative Study
Nano-to-micro scale dynamics of P-selectin detachment from leukocyte interfaces. III. Numerical simulation of tethering under flow
Michael R King et al. Biophys J. 2005 Mar.
Abstract
Transient capture of cells or model microspheres from flow over substrates sparsely coated with adhesive ligands has provided significant insight into the unbinding kinetics of leukocyte:endothelium adhesion complexes under external force. Whenever a cell is stopped by a point attachment, the full hydrodynamic load is applied to the adhesion site within an exceptionally short time-less than the reciprocal of the hydrodynamic shear rate (e.g., typically <0.01 s). The decay in numbers of cells or beads that remain attached to a surface has been used as a measure of the kinetics of molecular bond dissociation under constant force, revealing a modest increase in detachment rate at growing applied shear stresses. On the other hand, when detached under steady ramps of force with mechanical probes (e.g., the atomic force microscope and biomembrane force probe), P-selectin:PSGL-1 adhesion bonds break at rates that increase enormously under rising force, yielding 100-fold faster off rates at force levels comparable to high shear. The comparatively weak effect of force on tether survival in flow chamber experiments could be explained by a possible partition of the load amongst several bonds. However, a comprehensive understanding of the difference in kinetic behavior requires us to also inspect other factors affecting the dynamics of attachment-force buildup, such as the interfacial compliance of all linkages supporting the adhesion complex. Here, combining the mechanical properties of the leukocyte interface measured in probe tests with single-bond kinetics and the kinetics of cytoskeletal dissociation, we show that for the leukocyte adhesion complex P-selectin:PSGL-1, a detailed adhesive dynamics simulation accurately reproduces the tethering behavior of cells observed in flow chambers. Surprisingly, a mixture of 10% single bonds and 90% dimeric bonds is sufficient to fully match the data of the P-selectin:PSGL-1 experiments, with the calculated decay in fraction of attached cells still appearing exponential.
Figures
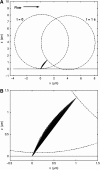
FIGURE 1
(A) Side-view outline of a tethered cell at a wall shear stress of 1.0 dyn/cm2, initially and after 1 s. The length and orientation of the tether linking the cell to the wall is plotted at intervals of 1/30 s. (B) Closeup of the bond region.
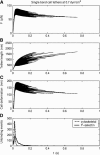
FIGURE 2
Time course of the bond force (A), tether length (B), and cell deformation (C) during individual cell tethering events at a wall shear stress of 0.7 dyn/cm2. In this case, tethers are mediated by single P-selectin:PSGL-1 bonds. Histograms of cytoskeletal and P-selectin:PSGL-1 dissociation events are given in panel D. Nine-hundred individual adhesion events are overlaid in panels A_–_C.
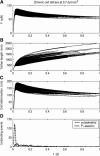
FIGURE 3
Time course of the bond force (A), tether length (B), and cell deformation (C) during individual cell tethering events at a wall shear stress of 0.7 dyn/cm2. In this case, tethers are mediated by dimeric P-selectin:PSGL-1 bonds that share force equally. Histograms of cytoskeletal and P-selectin:PSGL-1 dissociation events are given in panel D. Four-hundred individual adhesion events are overlaid in panels A_–_C.
FIGURE 4
Pause-time distributions used to calculate the “apparent” bond dissociation constants of neutrophils interacting with a P-selectin substrate. Simulated data for either single-bond tethers (A) or double-bond tethers (B) at different wall shear stresses are presented in the same form as in the experimental study of Park et al. (2002).
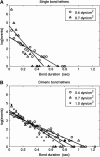
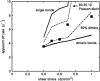
FIGURE 5
Dependence of the apparent off rate of the P-selectin:PSGL-1 bond on wall shear stress, as determined from the interpretation of real or simulated tethering experiments. Squares denote the experimental data of Park et al. (2002), and are compared with simulation results for either single or double bonds. Note that a mixture of single and double bonds, with a double-bond fraction of 90% agrees well with the experiments of Park et al. (2002). Also plotted for comparison is a relative weighting of 60% single bonds, 30% double bonds, and 10% triple bonds, corresponding to a Poisson distribution in bond number.
Similar articles
- Nano- to microscale dynamics of P-selectin detachment from leukocyte interfaces. II. Tether flow terminated by P-selectin dissociation from PSGL-1.
Heinrich V, Leung A, Evans E. Heinrich V, et al. Biophys J. 2005 Mar;88(3):2299-308. doi: 10.1529/biophysj.104.051706. Epub 2005 Jan 14. Biophys J. 2005. PMID: 15653735 Free PMC article. - Nano- to microscale dynamics of P-selectin detachment from leukocyte interfaces. I. Membrane separation from the cytoskeleton.
Evans E, Heinrich V, Leung A, Kinoshita K. Evans E, et al. Biophys J. 2005 Mar;88(3):2288-98. doi: 10.1529/biophysj.104.051698. Epub 2005 Jan 14. Biophys J. 2005. PMID: 15653718 Free PMC article. - Neutrophil-bead collision assay: pharmacologically induced changes in membrane mechanics regulate the PSGL-1/P-selectin adhesion lifetime.
Edmondson KE, Denney WS, Diamond SL. Edmondson KE, et al. Biophys J. 2005 Nov;89(5):3603-14. doi: 10.1529/biophysj.105.066134. Epub 2005 Aug 12. Biophys J. 2005. PMID: 16100264 Free PMC article. - Neutrophil rolling at high shear: flattening, catch bond behavior, tethers and slings.
Sundd P, Pospieszalska MK, Ley K. Sundd P, et al. Mol Immunol. 2013 Aug;55(1):59-69. doi: 10.1016/j.molimm.2012.10.025. Epub 2012 Nov 9. Mol Immunol. 2013. PMID: 23141302 Free PMC article. Review. - Mechanisms for flow-enhanced cell adhesion.
Zhu C, Yago T, Lou J, Zarnitsyna VI, McEver RP. Zhu C, et al. Ann Biomed Eng. 2008 Apr;36(4):604-21. doi: 10.1007/s10439-008-9464-5. Epub 2008 Feb 26. Ann Biomed Eng. 2008. PMID: 18299992 Free PMC article. Review.
Cited by
- Uncoiling mechanics of Escherichia coli type I fimbriae are optimized for catch bonds.
Forero M, Yakovenko O, Sokurenko EV, Thomas WE, Vogel V. Forero M, et al. PLoS Biol. 2006 Sep;4(9):e298. doi: 10.1371/journal.pbio.0040298. PLoS Biol. 2006. PMID: 16933977 Free PMC article. - Nano- to microscale dynamics of P-selectin detachment from leukocyte interfaces. II. Tether flow terminated by P-selectin dissociation from PSGL-1.
Heinrich V, Leung A, Evans E. Heinrich V, et al. Biophys J. 2005 Mar;88(3):2299-308. doi: 10.1529/biophysj.104.051706. Epub 2005 Jan 14. Biophys J. 2005. PMID: 15653735 Free PMC article. - Sticking to the Problem: Engineering Adhesion in Molecular Endoscopic Imaging.
Noori MS, Bodle SJ, Showalter CA, Streator ES, Drozek DS, Burdick MM, Goetz DJ. Noori MS, et al. Cell Mol Bioeng. 2020 Jan 21;13(2):113-124. doi: 10.1007/s12195-020-00609-0. eCollection 2020 Apr. Cell Mol Bioeng. 2020. PMID: 32175025 Free PMC article. Review. - Simulation and Analysis of Tethering Behavior of Neutrophils with Pseudopods.
Rocheleau AD, Sumagin R, Sarelius IH, King MR. Rocheleau AD, et al. PLoS One. 2015 Jun 19;10(6):e0128378. doi: 10.1371/journal.pone.0128378. eCollection 2015. PLoS One. 2015. PMID: 26091091 Free PMC article. - Biomechanics of leukocyte rolling.
Sundd P, Pospieszalska MK, Cheung LS, Konstantopoulos K, Ley K. Sundd P, et al. Biorheology. 2011;48(1):1-35. doi: 10.3233/BIR-2011-0579. Biorheology. 2011. PMID: 21515934 Free PMC article. Review.
References
- Alon, R., D. A. Hammer, and T. A. Springer. 1995. Lifetime of the P-selectin-carbohydrate bond and its response to tensile force in hydrodynamic flow. Nature. 374:539–542. - PubMed
- Bruehl, R. E., T. A. Springer, and D. F. Bainton. 1996. Quantitation of L-selectin distribution on human leukocyte microvilli by immunogold labeling and electron microscopy. J. Histochem. Cytochem. 44:835–844. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources