Chronic exposure to rotenone models sporadic Parkinson's disease in Drosophila melanogaster - PubMed (original) (raw)
Chronic exposure to rotenone models sporadic Parkinson's disease in Drosophila melanogaster
Hélène Coulom et al. J Neurosci. 2004.
Abstract
Parkinson's disease (PD) is a movement disorder characterized by the selective degeneration of nigrostriatal dopaminergic neurons. Both familial and sporadic cases present tremor, rigidity, slowness of movement, and postural instability. Although major insights into the genes responsible for some rare hereditary cases have arisen, the etiology of sporadic cases remains unknown. Epidemiological studies have suggested an association with environmental toxins, mainly mitochondrial complex I inhibitors such as the widely used pesticide rotenone. In recent years, Drosophila melanogaster has been used as a model of several neurodegenerative diseases, including a genetic model of PD. Here, we studied the neurodegenerative and behavioral effects of a sublethal chronic exposure to rotenone in Drosophila. After several days, the treated flies presented characteristic locomotor impairments that increased with the dose of rotenone. Immunocytochemistry analysis demonstrated a dramatic and selective loss of dopaminergic neurons in all of the brain clusters. The addition of l-dopa (3,4-dihydroxy-L-phenylalanine) into the feeding medium rescued the behavioral deficits but not neuronal death, as is the case in human PD patients. In contrast, the antioxidant melatonin (N-acetyl-5-methoxytryptamine) alleviated both symptomatic impairment and neuronal loss, supporting the idea that this agent may be beneficial in the treatment of PD. Therefore, chronic exposure to pesticides recapitulates key aspects of PD in Drosophila and provides a new in vivo model for studying the mechanisms of dopaminergic neurodegeneration.
Figures
Figure 1.
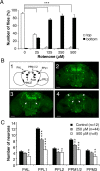
Exposure of Drosophila to rotenone induces severe locomotor deficits and dopaminergic neuron loss. A, Negative geotaxis assay of adult flies exposed previously for 7 d to various amounts of rotenone. White bars indicate the percentage of flies that climbed to the top of the column, and black bars indicate the percentage of flies that remained at the bottom after 1 min. Differences in PI between control and rotenone-treated flies were highly significant (***p < 0.001). B1, Schematic representation of the dopaminergic neuron clusters in Drosophila adult brain in frontal view. _B2_-B4, Tyrosine hydroxylase immunolabeling showing dopaminergic neuron patterns in multifocal confocal views of adult fly brains after 7 d of exposure to 0 (control), 250, and 500 μ
m
rotenone, respectively. White arrows indicate the total absence of certain clusters after rotenone exposure (PPM3 in B3 and PAM and PAL in B4). Scale bars, 50 μm. C, Quantification of the number of neurons in dopaminergic clusters of control brains (black bars) or in brains of flies exposed for 7 d to 250 μ
m
(gray bars) or 500 μ
m
(white bars) rotenone. The density of the neurons in the PAM clusters was too high to allow precise scoring of their number. n indicates the number of brain hemispheres examined in each condition. *p < 0.05, **p < 0.01, and ***p < 0.001 compared with control values.
Figure 2.
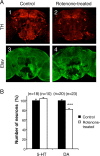
Selectivity of the neuronal degeneration induced by rotenone. A, Coimmunolabeling of adult brains with anti-tyrosine hydroxylase (TH) (A1, A2) and anti-Elav (A3, A4) antibodies after 7 d of exposure to 125 μ
m
rotenone. The pesticide induced obvious dopaminergic neuron loss, whereas the general pattern of the pan-neuronal Elav marker was not modified. B, Total number of serotoninergic (5-HT) and dopaminergic (DA) neurons in fly brains exposed for 7 d to 125 μ
m
rotenone (white bars) compared with control brains (black bars), expressed as a percentage of the mean value of the control. n indicates the number of brain hemispheres examined in each condition. ***p < 0.001.
Figure 3.
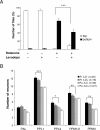
l
-Dopa improves locomotor phenotype but does not lessen dopaminergic neuron loss. A, Negative geotaxis assay of adult flies exposed previously for 7 d to 125 μ
m
rotenone, 1 m
m l
-dopa, or both, compared with control flies maintained in the absence of drugs. Representation and statistics are the same as in Figure 1 A. B, Quantification of neurons in control brains and in brains of flies exposed for 7 d to 125 μ
m
rotenone (R) with or without 1 m
m l
-dopa (LD). The number of dopaminergic neurons and the loss of cells as a result of rotenone exposure were not significantly altered by
l
-dopa. n indicates the number of brain hemispheres examined in each condition. Statistics are the same as in Figure 1_C_.
Figure 4.
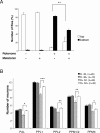
Melatonin improves locomotor deficits and protects dopaminergic neurons. A, Negative geotaxis assay of adult flies exposed previously for 7 d to 125 μ
m
rotenone, 5 m
m
melatonin, or both, compared with control flies maintained in the absence of drugs. Representation is the same as that in Figure 1 A. B, Quantification of dopaminergic neurons in brains of control flies exposed or not exposed for 7 d to 5 m
m
melatonin (M) and in flies exposed for 7 d to 125 μ
m
rotenone (R) with or without 5 m
m
melatonin. n indicates the number of brain hemispheres examined in each condition. *p < 0.05, **p < 0.01, and ***p < 0.001 compared with flies exposed to rotenone without melatonin.
Similar articles
- Mechanism of toxicity in rotenone models of Parkinson's disease.
Sherer TB, Betarbet R, Testa CM, Seo BB, Richardson JR, Kim JH, Miller GW, Yagi T, Matsuno-Yagi A, Greenamyre JT. Sherer TB, et al. J Neurosci. 2003 Nov 26;23(34):10756-64. doi: 10.1523/JNEUROSCI.23-34-10756.2003. J Neurosci. 2003. PMID: 14645467 Free PMC article. - Ameliorative effects of flavonoids and polyketides on the rotenone induced Drosophila model of Parkinson's disease.
Siima AA, Stephano F, Munissi JJE, Nyandoro SS. Siima AA, et al. Neurotoxicology. 2020 Dec;81:209-215. doi: 10.1016/j.neuro.2020.09.004. Epub 2020 Sep 13. Neurotoxicology. 2020. PMID: 32937168 - Intersecting pathways to neurodegeneration in Parkinson's disease: effects of the pesticide rotenone on DJ-1, alpha-synuclein, and the ubiquitin-proteasome system.
Betarbet R, Canet-Aviles RM, Sherer TB, Mastroberardino PG, McLendon C, Kim JH, Lund S, Na HM, Taylor G, Bence NF, Kopito R, Seo BB, Yagi T, Yagi A, Klinefelter G, Cookson MR, Greenamyre JT. Betarbet R, et al. Neurobiol Dis. 2006 May;22(2):404-20. doi: 10.1016/j.nbd.2005.12.003. Epub 2006 Jan 24. Neurobiol Dis. 2006. PMID: 16439141 - Paraquat- and rotenone-induced models of Parkinson's disease.
Nisticò R, Mehdawy B, Piccirilli S, Mercuri N. Nisticò R, et al. Int J Immunopathol Pharmacol. 2011 Apr-Jun;24(2):313-22. doi: 10.1177/039463201102400205. Int J Immunopathol Pharmacol. 2011. PMID: 21658306 Review. - Effect of Rotenone on the Neurodegeneration among Different Models.
Subhan I, Siddique YH. Subhan I, et al. Curr Drug Targets. 2024;25(8):530-542. doi: 10.2174/0113894501281496231226070459. Curr Drug Targets. 2024. PMID: 38698744 Review.
Cited by
- A microfluidic phenotype analysis system reveals function of sensory and dopaminergic neuron signaling in C. elegans electrotactic swimming behavior.
Salam S, Ansari A, Amon S, Rezai P, Selvaganapathy PR, Mishra RK, Gupta BP. Salam S, et al. Worm. 2013 Apr 1;2(2):e24558. doi: 10.4161/worm.24558. Epub 2013 Apr 18. Worm. 2013. PMID: 24058875 Free PMC article. - A standardized method for incorporation of drugs into food for use with Drosophila melanogaster.
Kruger L, Denton TT. Kruger L, et al. Anal Biochem. 2020 Jun 15;599:113740. doi: 10.1016/j.ab.2020.113740. Epub 2020 Apr 19. Anal Biochem. 2020. PMID: 32320689 Free PMC article. - A Drosophila model of the neurodegenerative disease SCA17 reveals a role of RBP-J/Su(H) in modulating the pathological outcome.
Ren J, Jegga AG, Zhang M, Deng J, Liu J, Gordon CB, Aronow BJ, Lu LJ, Zhang B, Ma J. Ren J, et al. Hum Mol Genet. 2011 Sep 1;20(17):3424-36. doi: 10.1093/hmg/ddr251. Epub 2011 Jun 8. Hum Mol Genet. 2011. PMID: 21653638 Free PMC article. - Anacardium microcarpum extract and fractions protect against paraquat-induced toxicity in Drosophila melanogaster.
Müller KR, Martins IK, Rodrigues NR, da Cruz LC, Barbosa Filho VM, Macedo GE, da Silva GF, Kamdem JP, de Menezes IRA, Franco JL, Posser T. Müller KR, et al. EXCLI J. 2017 Mar 20;16:302-312. doi: 10.17179/excli2016-684. eCollection 2017. EXCLI J. 2017. PMID: 28507474 Free PMC article.
References
- Antolin I, Mayo JC, Sainz RM, del Brio Mde L, Herrera F, Martin V, Rodriguez C (2002) Protective effect of melatonin in a chronic experimental model of Parkinson's disease. Brain Res 943: 163-173. - PubMed
- Auluck PK, Chan HY, Trojanowski JQ, Lee VM, Bonini NM (2002) Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson's disease. Science 295: 865-868. - PubMed
- Beal MF (2001) Experimental models of Parkinson's disease. Nat Rev Neurosci 2: 325-334. - PubMed
- Beal MF (2003) Mitochondria, oxidative damage, and inflammation in Parkinson's disease. Ann NY Acad Sci 991: 120-131. - PubMed
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT (2000) Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci 3: 1301-1306. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases