Beta1-integrin orients epithelial polarity via Rac1 and laminin - PubMed (original) (raw)
Beta1-integrin orients epithelial polarity via Rac1 and laminin
Wei Yu et al. Mol Biol Cell. 2005 Feb.
Abstract
Epithelial cells polarize and orient polarity in response to cell-cell and cell-matrix adhesion. Although there has been much recent progress in understanding the general polarizing machinery of epithelia, it is largely unclear how this machinery is controlled by the extracellular environment. To explore the signals from cell-matrix interactions that control orientation of cell polarity, we have used three-dimensional culture systems in which Madin-Darby canine kidney (MDCK) cells form polarized, lumen-containing structures. We show that interaction of collagen I with apical beta1-integrins after collagen overlay of a polarized MDCK monolayer induces activation of Rac1, which is required for collagen overlay-induced tubulocyst formation. Cysts, comprised of a monolayer enclosing a central lumen, form after embedding single cells in collagen. In those cultures, addition of a beta1-integrin function-blocking antibody to the collagen matrix gives rise to cysts that have defects in the organization of laminin into the basement membrane and have inverted polarity. Normal polarity is restored by either expression of activated Rac1, or the inclusion of excess laminin-1 (LN-1). Together, our results suggest a signaling pathway in which the activation of beta1-integrins orients the apical pole of polarized cysts via a mechanism that requires Rac1 activation and laminin organization into the basement membrane.
Figures
Figure 1.
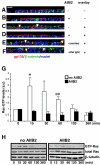
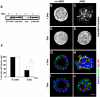
β1 integrin-mediated activation of Rac1 is required for the formation of tubulocysts upon collagen overlay. (A-F) Confocal images of gp135 (red) and β-catenin (green) of vertical sections show MDCK cells grown on Transwell filters at indicated conditions. (A) Confluent MDCK in the absence of AIIB2 and collagen gel have a polarized monolayer morphology. (B) Treatment with 8 μg/ml AIIB2 for 5 d does not change the monolayer phenotype. (C) MDCK cells overlaid on the apical surface with type I collagen gel for 5 d induces the formation of tubulocysts. (D) Addition of AIIB2 to the collagen inhibits tubulocyst formation in response to apical collagen overlay. (E and F) Control Rat IgG and mouse ascites 9E10 do not inhibit tubulocyst formation in response to collagen overlay. Arrowheads indicate lumens. (G) Time course of Rac1 activation by collagen overlay in the absence (white column) or presence (black column) of AIIB2 was assessed by a GST-CRIB-PAK pull-down assay. The apical surface of a polarized MDCK monolayer was overlaid with collagen for the indicated periods. Results were normalized to GTP-Rac1 levels in the 0-min lane. The graph represents five independent experiments ± SD. *p < 0.03, **p < 0.01, relative to no overlay (0 min). (H) Representative Western blots of Rac1 activation for Figure 1G, β-tubulin serves as loading control. Bar, 10 μm.
Figure 2.
Rac1 activation is required for tubulocyst formation. (A) Endogenous and mutant Rac1 protein levels and Rac1 activation in cells that express myc-tagged N17Rac1 and V12Rac1 under control of the tetracycline-repressible transactivator. MDCK monolayers grown on filters in the absence or presence of Dox were overlaid with collagen gel for the indicated times. Western blots show total Rac1 expression. Double bands in total lysates of mutant Rac1-expressing cells (-Dox) show expression of endogenous (top band) and myc-tagged mutant Rac1 (bottom band). Activated Rac1 (GTP-Rac1) was determined by a GST-CRIB-PAK pull-down assay. GTP-Rac1 was not detected in cells expressing N17Rac1, but it was high in V12Rac1 cells. β-Tubulin was used as a loading control. (B) Vertical sections of monolayers stained for gp135 (red) and β-catenin (green) show mutant Rac1-expressing cells grown with or without collagen overlay and AIIB2 as indicated. Both noninduced and induced N17Rac1 cells remain a polarized monolayer in the absence of collagen overlay (2B-1 and 2B-2). Noninduced N17Rac1 cells develop normal tubulocysts (2B-3), whereas N17Rac1-expressing cells do not form tubulocysts (2B-4). Both noninduced V12Rac1 and induced V12Rac1 cells have polarized monolayer phenotypes in the absence of collagen overlay (2B-5 and 2B-6) and form tubulocysts in the presence of collagen overlay (2B-7 and 2B-8). When AIIB2 is present in the collagen overlay, tubulocyst formation is blocked in noninduced V12Rac1 cells (2B-9), but not in cells expressing V12Rac1 (2B-10). Arrowheads indicate lumens. Bar, 10 μm.
Figure 3.
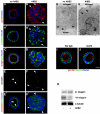
Inhibition of β1 integrins inverts apical polarity during cystogenesis and reduces expression of α3 integrin. (A-F) Cysts, grown in the absence (A, A′, C, E, and E′) or presence (B, B′, D, F, and F′) of 8 μg/ml AIIB2 were stained for markers of the apical pole (gp135, Golgi, and occludin) in red, for the basolateral membrane marker β-catenin (A′ and B′) or actin (C, D, E′, and F′) in green and for nuclei in blue. The tight junctional marker occludin is stained red in E′ and F′, and is shown alone for clarity in E and F. In untreated cysts, the apical marker gp135 (A), the Golgi marker GM130 (C), and occludin (E and E′) are localized at or toward the interior luminal surface, whereas staining of the basolateral membrane marker β-catenin (A′) is limited to cell-cell contacts and the peripheral surface and is excluded from the luminal surface (A and A′ represent the same cyst). Moreover, intense actin staining is observed around the lumen (C and E′). These staining patterns indicate a normal polarization. In contrast, polarity in AIIB2-treated cysts is inverted, as indicated by peripheral staining of gp135 (B), exclusion of β-catenin from peripheral surface (B′), orientation of the Golgi underneath the peripheral surface (D), and peripheral staining of occludin (F and F′). Arrows show that gp135 occurs at peripheral surface and β-catenin disappears from these areas in AIIB2-treated cysts (B and B′). Arrowheads point to junctional complexes, which are facing the lumen in control cysts (E and E′), and the periphery in AIIB2-treated cysts (F and F′). (G and H) Electron micrographs of cysts. In cysts grown in absence of AIIB2, tight junctions (arrowheads) at cell-cell contacts are close to the lumen and microvilli (arrow) face the lumen. In the presence of AIIB2, junctions face the collagen. (I) Addition of rat IgG as a control antibody does not affect cystogenesis. (J) Treatment with rat anti-integrin α6 antibody does not change the phenotype of cysts. (K) Western blot analysis shows that β1 integrin levels are not changed (bottom band of doublet represents a precursor form of β1 integrin), but α3 integrin is decreased in AIIB2-treated cysts. β-Tubulin levels are used as loading control. Bars, 10 μm (A-F, I, and J) and 2 μm (G and H).
Figure 5.
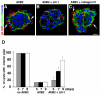
Exogenous LN-1 rescues the normal cyst phenotype. (A-C) Cysts grown in the presence of AIIB2 have inverted polarity (A). Addition of exogenous LN-1 (B) but not collagen IV (C) in AIIB2-treated cysts reverts the apical pole to the luminal surface and induces the formation of lumens. Confocal images represent 7-d-old AIIB2-treated cysts stained with the apical protein marker gp135 (red), basolateral protein, β-catenin (green), and nuclei (blue) in the presence of LN-1 or collagen IV. Arrows in A and C indicate the presence of the apical gp135 at the periphery of the cysts. (D) Quantitation of cyst phenotypes in untreated cysts, AIIB2-treated cysts, and AIIB2-treated cysts with exogenous LN-1 shows that in the presence of exogenous LN-1, the proportion of cysts with an interior pole gradually increases over time. Bar, 10 μm (A-C).
Figure 4.
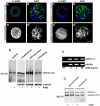
AIIB2-treated cysts have disorganized deposition of laminin and collagen IV. (A-H) Immunofluorescent staining of laminin (red in A and B), collagen IV (red in C and D), actin (green), and nuclei (blue) reveal the fine network of laminin and collagen IV on the peripheral surface of untreated cysts (A and C), but large aggregates in AIIB2-treated cysts (B and D). Projections of serial confocal sections more clearly demonstrate this difference between untreated (A′, laminin and C′, collagen IV) and AIIB2-treated cysts (B′, laminin and D′, collagen IV). (E) Western blotting of immunoprecipitated laminin shows no difference in secreted laminin (medium), total synthesized laminin (solubilized gel), cell-associated laminin (isolated cysts), and intracellular laminin (isolated and trypsin-treated cysts) between untreated and AIIB2-treated cysts. The β1/γ1 bands migrated at ∼200 kDa. (F) RT-PCR analysis shows that treatment with AIIB2 does not significantly affect the expression levels of laminin α5 chains. RT is control without reverse transcriptase. (G) Untreated or AIIB2-treated cysts were metabolically labeled with
l
-[35S]methionine. Micrograph shows autoradiogram of laminin immunoprecipitated from isolated cysts, representing intracellular and cell-associated extracellular laminin, and from isolated cysts treated with trypsin to digest extracellular laminin (intracellular). In addition tot the β1/γ1 chains ∼200 kDa, an ∼400-kDa protein was immunoprecipitated, which likely respresents a coimmunoprecipitating laminin α chain from a β1/γ1-containing laminin heterotrimer. Note that AIIB2 does not affect levels of any laminin chain or ratio between the α and the β1/γ1 chains. Bar (A-D′), 10 μm.
Figure 6.
Expression of constitutively active Rac1 induces the establishment of an interior apical pole in the presence of AIIB2. (A) Expression of endogenous and exogenous Rac1 and GTP-Rac1 in MDCK cysts that express V12Rac1 under control of a tetracycline-repressible transactivator. Cysts, grown in the presence or absence of Dox, were cultured for 7 d and Rac activation was analyzed as described in Materials and Methods. Levels of endogenous Rac1 and the myc-tagged V12Rac1 mutant were detected by anti-Rac1 and anti-myc antibody. β-Tubulin staining was used as loading control. Note that Rac-GTP levels are below background in noninduced controls (+Dox) and high in V12Rac1-expressing cysts (-Dox). (B-E) Projection of multiple confocal images of V12Rac1-expressing cysts stained for laminin. Noninduced V12Rac1 cells have uniform laminin deposition in untreated cysts (B) and irregular laminin deposition when grown in the presence of AIIB2 (C). V12Rac1 expressing cysts have uniform laminin distribution, in both untreated (D) and AIIB2-treated (E) cultures. (F) Quantitation of V12Rac1 cyst phenotypes, as described in Materials and Methods, indicates that 99% of either noninduced or V12Rac1-expressing cysts have normal polarity. In the presence of AIIB2, only 30% of noninduced cysts have normal polarity, whereas expression of V12Rac1 rescues the normal polarity and increases the percentage of cysts with an interior apical pole to 85% of the total population. The graphs represent the means ± SD of three separate experiments. In each experiment, 200 cysts per condition were analyzed. (G-J) Representative confocal images for F. Staining for gp135 (red), β-catenin (green), and nuclei (blue) shows that V12Rac1 cells form cysts with normal polarity (G) and cysts treated with AIIB2 have a peripheral apical pole (H) as described in Figure 3. V12Rac1-expressing cells form normal cysts with gp135 at the luminal surface, in the absence of AIIB2 (I). In the presence of AIIB2, V12Rac1-expressing cells still form cysts with an interior apical pole (J), although the lumen is not as well established as in untreated cysts. Bar, 10 μm.
Similar articles
- Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly.
O'Brien LE, Jou TS, Pollack AL, Zhang Q, Hansen SH, Yurchenco P, Mostov KE. O'Brien LE, et al. Nat Cell Biol. 2001 Sep;3(9):831-8. doi: 10.1038/ncb0901-831. Nat Cell Biol. 2001. PMID: 11533663 - Formation of multicellular epithelial structures.
Mostov K, Brakeman P, Datta A, Gassama A, Katz L, Kim M, Leroy P, Levin M, Liu K, Martin F, O'Brien LE, Verges M, Su T, Tang K, Tanimizu N, Yamaji T, Yu W. Mostov K, et al. Novartis Found Symp. 2005;269:193-200; discussion 200-5, 223-30. Novartis Found Symp. 2005. PMID: 16355541 - Apical beta 1 integrin in polarized MDCK cells mediates tubulocyst formation in response to type I collagen overlay.
Zuk A, Matlin KS. Zuk A, et al. J Cell Sci. 1996 Jul;109 ( Pt 7):1875-89. doi: 10.1242/jcs.109.7.1875. J Cell Sci. 1996. PMID: 8832410 - Integrins in epithelial cell polarity: using antibodies to analyze adhesive function and morphogenesis.
Matlin KS, Haus B, Zuk A. Matlin KS, et al. Methods. 2003 Jul;30(3):235-46. doi: 10.1016/s1046-2023(03)00030-6. Methods. 2003. PMID: 12798138 Review. - Laminins in Epithelial Cell Polarization: Old Questions in Search of New Answers.
Matlin KS, Myllymäki SM, Manninen A. Matlin KS, et al. Cold Spring Harb Perspect Biol. 2017 Oct 3;9(10):a027920. doi: 10.1101/cshperspect.a027920. Cold Spring Harb Perspect Biol. 2017. PMID: 28159878 Free PMC article. Review.
Cited by
- Rotational motion during three-dimensional morphogenesis of mammary epithelial acini relates to laminin matrix assembly.
Wang H, Lacoche S, Huang L, Xue B, Muthuswamy SK. Wang H, et al. Proc Natl Acad Sci U S A. 2013 Jan 2;110(1):163-8. doi: 10.1073/pnas.1201141110. Epub 2012 Dec 17. Proc Natl Acad Sci U S A. 2013. PMID: 23248267 Free PMC article. - Bioengineering in salivary gland regeneration.
Hajiabbas M, D'Agostino C, Simińska-Stanny J, Tran SD, Shavandi A, Delporte C. Hajiabbas M, et al. J Biomed Sci. 2022 Jun 6;29(1):35. doi: 10.1186/s12929-022-00819-w. J Biomed Sci. 2022. PMID: 35668440 Free PMC article. Review. - Noncanonical frizzled signaling regulates cell polarity of growth plate chondrocytes.
Li Y, Dudley AT. Li Y, et al. Development. 2009 Apr;136(7):1083-92. doi: 10.1242/dev.023820. Epub 2009 Feb 18. Development. 2009. PMID: 19224985 Free PMC article. - The PI 3-kinase and mTOR signaling pathways are important modulators of epithelial tubule formation.
Walid S, Eisen R, Ratcliffe DR, Dai K, Hussain MM, Ojakian GK. Walid S, et al. J Cell Physiol. 2008 Aug;216(2):469-79. doi: 10.1002/jcp.21419. J Cell Physiol. 2008. PMID: 18366086 Free PMC article. - Tunable Hybrid Matrices Drive Epithelial Morphogenesis and YAP Translocation.
Zhang Y, Zegers MMP, Nagelkerke A, Rowan AE, Span PN, Kouwer PHJ. Zhang Y, et al. Adv Sci (Weinh). 2020 Dec 11;8(2):2003380. doi: 10.1002/advs.202003380. eCollection 2021 Jan. Adv Sci (Weinh). 2020. PMID: 33511022 Free PMC article.
References
- Aumailley, M., Pesch, M., Tunggal, L., Gaill, F., and Fassler, R. (2000). Altered synthesis of laminin 1 and absence of basement membrane component deposition in (beta)1 integrin-deficient embryoid bodies. J. Cell Sci. 113, 259-268. - PubMed
- Caplan, M. J., Stow, J. L., Newman, A. P., Madri, J., Anderson, H. C., Farquhar, M. G., Palade, G. E., and Jamieson, J. D. (1987). Dependence on pH of polarized sorting of secreted proteins. Nature 329, 632-635. - PubMed
- Colognato, H., and Yurchenco, P. D. (2000). Form and function: the laminin family of heterotrimers. Dev. Dyn. 218, 213-234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HD046768/HD/NICHD NIH HHS/United States
- T32 DK064581/DK/NIDDK NIH HHS/United States
- R01 DK046768/DK/NIDDK NIH HHS/United States
- T32 DK64581/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials