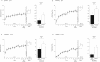Sleep-dependent learning and motor-skill complexity - PubMed (original) (raw)
Clinical Trial
. 2004 Nov-Dec;11(6):705-13.
doi: 10.1101/lm.76304.
Affiliations
- PMID: 15576888
- PMCID: PMC534699
- DOI: 10.1101/lm.76304
Clinical Trial
Sleep-dependent learning and motor-skill complexity
Kenichi Kuriyama et al. Learn Mem. 2004 Nov-Dec.
Abstract
Learning of a procedural motor-skill task is known to progress through a series of unique memory stages. Performance initially improves during training, and continues to improve, without further rehearsal, across subsequent periods of sleep. Here, we investigate how this delayed sleep-dependent learning is affected when the task characteristics are varied across several degrees of difficulty, and whether this improvement differentially enhances individual transitions of the motor-sequence pattern being learned. We report that subjects show similar overnight improvements in speed whether learning a five-element unimanual sequence (17.7% improvement), a nine-element unimanual sequence (20.2%), or a five-element bimanual sequence (17.5%), but show markedly increased overnight improvement (28.9%) with a nine-element bimanual sequence. In addition, individual transitions within the motor-sequence pattern that appeared most difficult at the end of training showed a significant 17.8% increase in speed overnight, whereas those transitions that were performed most rapidly at the end of training showed only a non-significant 1.4% improvement. Together, these findings suggest that the sleep-dependent learning process selectively provides maximum benefit to motor-skill procedures that proved to be most difficult prior to sleep.
Figures
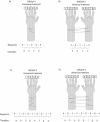
Figure 4.
Motor-skill task configurations representing each of the four different experimental groups. The four different task configurations (corresponding to the four experimental groups) were varied across two different task characteristics - the limb complexity and the sequence length. (A) Group 1 performed a unimanual five-element configuration (four fingers, using one hand), (B) Group 2 performed a bimanual five-element configuration (four fingers, using two hands), (C) Group 3 performed a unimanual nine-element configuration (four fingers, using one hand), and (D) Group 4 performed a bimanual nine-element configuration (eight fingers, using two hands). For each sequence, individual transitions between successive key-press responses were mapped alphabetically, providing a within-sequence transition profile for each subject in each of the respective groups.
Figure 1.
Differences in motor-skill speed across groups 1-4 during training and during retesting following sleep. Line graphs representing performance during initial training (trials 1-12, ♦), and during the three retest trials (R1-3, ⋄) for each of the four experimental groups (1-4; A,B,C,D, respectively). The fitted line of each graph represents the predicted (extrapolated) improvement rate based on the logarithmic regression across the 12 trials of training. Each line graph is accompanied by a corresponding summary bar graph representing the comparison between posttraining score (average of final three trials of training, open bar), and the retest score (average of three retest trials, filled bar). Error bars, SEM; Asterisks represent significance (P) compared with previous time point at: (*) < 0.05; (**) < 0.005.
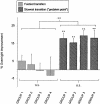
Figure 2.
Group difference in the overnight percentage improvement in transition speed occurring at the fastest and slowest transition positions. Prior to sleep, the fastest transition positions and slowest transition positions at the end of training were identified for each subject in each group, and following a night of sleep, the percentage change at each of these same two transitions positions was measured. A clear dissociation in overnight improvement was evident, with all groups exhibiting a consistent and large improvement following sleep for the slowest transition (light gray bars, problem point), but no group showing significant improvement for the fastest transition (dark-hatched gray bars). Error bars, SEM; Asterisks represent significance (P): (**) < 0.005; (n.s.) nonsignificant.
Figure 3.
Representative single-subject examples of the pre- and postsleep transition profiles within sequences for each of the four groups. For an individual subject, in each of the groups (1-4, A,B,C,D, respectively), the pre-sleep transition profile (○) and the post-sleep transition profile (•) was calculated within each sequence. Pre-sleep profiles demonstrated considerable variability, with certain transitions being particularly slow (most difficult; problem points), whereas other transitions appear to be relatively rapid (easy). Yet, a prominent feature following a night of sleep, independent of the group/task, was a specific reduction (improvement) in the speed of the slowest problem-point transition, whereas the faster transitions prior to sleep showed a lack of consistent improvement following sleep.
Similar articles
- Selective delayed gains following motor imagery of complex movements.
Debarnot U, Castellani E, Guillot A. Debarnot U, et al. Arch Ital Biol. 2012 Dec;150(4):238-50. doi: 10.4449/aib.v150i4.1394. Arch Ital Biol. 2012. PMID: 23479457 - Practice with sleep makes perfect: sleep-dependent motor skill learning.
Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Walker MP, et al. Neuron. 2002 Jul 3;35(1):205-11. doi: 10.1016/s0896-6273(02)00746-8. Neuron. 2002. PMID: 12123620 - Offline consolidation in implicit sequence learning.
Meier B, Cock J. Meier B, et al. Cortex. 2014 Aug;57:156-66. doi: 10.1016/j.cortex.2014.03.009. Epub 2014 Apr 5. Cortex. 2014. PMID: 24861420 - Does sleep promote motor learning? Implications for physical rehabilitation.
Siengsukon CF, Boyd LA. Siengsukon CF, et al. Phys Ther. 2009 Apr;89(4):370-83. doi: 10.2522/ptj.20080310. Epub 2009 Feb 6. Phys Ther. 2009. PMID: 19201986 Review. - Sleep and motor skill learning.
Laureys S, Peigneux P, Perrin F, Maquet P. Laureys S, et al. Neuron. 2002 Jul 3;35(1):5-7. doi: 10.1016/s0896-6273(02)00766-3. Neuron. 2002. PMID: 12123601 Review.
Cited by
- An N-methyl-D-aspartate receptor agonist facilitates sleep-independent synaptic plasticity associated with working memory capacity enhancement.
Kuriyama K, Honma M, Shimazaki M, Horie M, Yoshiike T, Koyama S, Kim Y. Kuriyama K, et al. Sci Rep. 2011;1:127. doi: 10.1038/srep00127. Epub 2011 Oct 24. Sci Rep. 2011. PMID: 22355644 Free PMC article. Clinical Trial. - Dynamics of sleep spindles and coupling to slow oscillations following motor learning in adult mice.
Kam K, Pettibone WD, Shim K, Chen RK, Varga AW. Kam K, et al. Neurobiol Learn Mem. 2019 Dec;166:107100. doi: 10.1016/j.nlm.2019.107100. Epub 2019 Oct 14. Neurobiol Learn Mem. 2019. PMID: 31622665 Free PMC article. - Human hippocampal replay during rest prioritizes weakly learned information and predicts memory performance.
Schapiro AC, McDevitt EA, Rogers TT, Mednick SC, Norman KA. Schapiro AC, et al. Nat Commun. 2018 Sep 25;9(1):3920. doi: 10.1038/s41467-018-06213-1. Nat Commun. 2018. PMID: 30254219 Free PMC article. - Performance-Dependent Consolidation of Learned Vocal Changes in Adult Songbirds.
Tachibana RO, Lee D, Kai K, Kojima S. Tachibana RO, et al. J Neurosci. 2022 Mar 9;42(10):1974-1986. doi: 10.1523/JNEUROSCI.1942-21.2021. Epub 2022 Jan 20. J Neurosci. 2022. PMID: 35058370 Free PMC article. - Young and Older Adults Benefit From Sleep, but Not From Active Wakefulness for Memory Consolidation of What-Where-When Naturalistic Events.
Abichou K, La Corte V, Hubert N, Orriols E, Gaston-Bellegarde A, Nicolas S, Piolino P. Abichou K, et al. Front Aging Neurosci. 2019 Mar 20;11:58. doi: 10.3389/fnagi.2019.00058. eCollection 2019. Front Aging Neurosci. 2019. PMID: 30949043 Free PMC article.
References
- Andres, F.G., Mima, T., Schulman, A.E., Dichgans, J., Hallett, M., and Gerloff, C. 1999. Functional coupling of human cortical sensorimotor areas during bimanual skill acquisition. Brain 122: 855-870. - PubMed
- Atienza, M., Cantero, J.L., and Stickgold, R. 2004. Posttraining sleep enhances automaticity in perceptual discrimination. J. Cogn. Neurosci. 16: 53-64. - PubMed
- Beisteiner, R., Windischberger, C., Lanzenberger, R., Edward, V., Cunnington, R., Erdler, M., Gartus, A., Streibl, B., Moser, E., and Deecke, L. 2001. Finger somatotopy in human motor cortex. Neuroimage 13: 1016-1026. - PubMed
- De Weerd, P., Reinke, K., Ryan, L., McIsaac, T., Perschler, P., Schnyer, D., Trouard, T., and Gmitro, A. 2003. Cortical mechanisms for acquisition and performance of bimanual motor sequences. Neuroimage 19: 1405-1416. - PubMed
Publication types
MeSH terms
Grants and funding
- R21 MH067754/MH/NIMH NIH HHS/United States
- MH67754/MH/NIMH NIH HHS/United States
- MH65292/MH/NIMH NIH HHS/United States
- MH48832/MH/NIMH NIH HHS/United States
- MH069935/MH/NIMH NIH HHS/United States
- R01 MH065292/MH/NIMH NIH HHS/United States
- R01 MH048832/MH/NIMH NIH HHS/United States
- R03 MH069935/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources