Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice - PubMed (original) (raw)
Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice
Despina Sitara et al. Matrix Biol. 2004 Nov.
Abstract
Fibroblast growth factor-23 (FGF-23), a recently identified molecule that is mutated in patients with autosomal dominant hypophosphatemic rickets (ADHR), appears to be involved in the regulation of phosphate homeostasis. Although increased levels of circulating FGF-23 were detected in patients with different phosphate-wasting disorders such as oncogenic osteomalacia (OOM) and X-linked hypophosphatemia (XLH), it is not yet clear whether FGF-23 is directly responsible for the abnormal regulation of mineral ion homeostasis and consequently bone development. To address some of these unresolved questions, we generated a mouse model, in which the entire Fgf-23 gene was replaced with the lacZ gene. Fgf-23 null (Fgf-23-/-) mice showed signs of growth retardation by day 17, developed severe hyperphosphatemia with elevated serum 1,25(OH)2D3 levels, and died by 13 weeks of age. Hyperphosphatemia in Fgf-23-/- mice was accompanied by skeletal abnormalities, as demonstrated by histological, molecular, and various other morphometric analyses. Fgf-23-/-) mice had increased total-body bone mineral content (BMC) but decreased bone mineral density (BMD) of the limbs. Overall, Fgf-23-/- mice exhibited increased mineralization, but also accumulation of unmineralized osteoid leading to marked limb deformities. Moreover, Fgf-23-/- mice showed excessive mineralization in soft tissues, including heart and kidney. To further expand our understanding regarding the role of Fgf-23 in phosphate homeostasis and skeletal mineralization, we crossed Fgf-23-/- animals with Hyp mice, the murine equivalent of XLH. Interestingly, Hyp males lacking both Fgf-23 alleles were indistinguishable from Fgf-23/-/ mice, both in terms of serum phosphate levels and skeletal changes, suggesting that Fgf-23 is upstream of the phosphate regulating gene with homologies to endopeptidases on the X chromosome (Phex) and that the increased plasma Fgf-23 levels in Hyp mice (and in XLH patients) may be at least partially responsible for the phosphate imbalance in this disorder.
Figures
Fig. 1
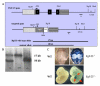
(A) Schematic representation of the murine Fgf-23 gene and the corresponding knock-out/in targeting vector. Exons 1 to 3 are shown in black boxes. Vertical and horizontal shaded boxes represent the 5′ and 3′ flanking regions of the Fgf-23 gene, respectively, which were used for homologous recombination. The lacZ gene was cloned in frame with the initiator methionine of the Fgf-23 gene. The neomycin resistance (neo) gene is driven by the phosphoglycerate kinase-1 (PGK-1) promoter and contains an Sv40 polyA adenylation site. Probe A was used as external probe to hybridize genomic Southern blots (_Bgl_II digest) shown in (B) (wild type=+/+, heterozygous=+/−, homozygous=−/−). Panel (C) represents lacZ staining of a wild-type (lower left) and a heterozygous Fgf-23 embryo (Fgf-23+/−, lower right) at E12.5. Arrows depict lacZ positive tissues (somites, liver, and heart). Upper panels demonstrate lacZ staining in a wild type (left) and _Fgf-23_−/− (right) skull at 3 weeks. Blue staining represents expression of the Fgf-23 gene.
Fig. 2
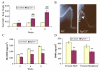
(A) Graphic display of total bone mineral content (BMC) of control and _Fgf-23_−/− animals at 3, 6, and 11 weeks. Each value obtained for BMC was normalized to the body weight of the corresponding animal. _Fgf-23_−/− mice show a statistical significant increase in total BMC when compared to control littermates (*=p<0.05; ***=p<0.0001). A statistically significant increase in total BMC was also observed among _Fgf-23_−/− mice with time (###=p<0.0001). (B) X-ray autoradiography of hindlimbs from a wild-type (WT) and an _Fgf-23_−/− mouse. Brackets depict length, and arrowhead depicts thickness of femur in _Fgf-23_−/− mouse. (C) Graph represents bone mineral density of hindlimbs measured by PIXImus analysis. _Fgf-23_−/− mice show a statistically significant decrease in BMD at 3, 6, and 11 weeks when compared to controls (***=p<0.0001). (D) BMD obtained from femoral shaft (left) and femoral methaphysis (right) of wild-type (white bar) and _Fgf-23_−/− animals (dark bar) by QCT at 4 weeks of age. _Fgf-23_−/− mice show a statistically significant decrease in BMD (**=p<0.001).
Fig. 3
(A) Alizarin red S staining of skeletal elements (ribs, paws, ulna/radius) from a wild type (left panels) and _Fgf-23_−/− (right panels) at 3 weeks. Arrows depict some areas with abnormal mineralization in _Fgf-23_−/− bones. (B) Abnormal mineralization is shown in heart (top) and in and around the tubules of _Fgf-23_−/− kidney (bottom) at 11 weeks. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 4
Three-micrometer-thick undecalcified sections from 4-week-old wild-type (upper panels) and _Fgf-23_−/− (lower panels) bones (cortical bone, growth plate, ribs, vertebra) were stained with von Kossa/McNeal (magnification 20×, 10×). Black staining represents mineralization. More mineral deposition is found in the area below the growth plate (methaphysis), ribs, and in vertebra. In contrast, areas of unmineralized osteoid (light blue) are found in cortical bone. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 5
In situ hybridization was performed on 6μm-thick decalcified paraffin sections from tibia of wild-type (WT) and _Fgf-23_−/− animals at 3 weeks. The zone of hypertrophic chondrocytes was reduced in _Fgf-23_−/−, which was confirmed by decreased collagen type X mRNA expression. In contrast, osteopontin mRNA expression was elevated in osteoblasts of _Fgf-23_−/− animals, while bone gla protein (osteocalcin) mRNA expression was diminished.
Fig. 6
(A) Gross features of three male littermates at 3 weeks. Shown are Hyp/Fgf23+/− (left), _Fgf23_−/− (right), and compound mutants _Hyp/Fgf23_−/− (middle). (B) X-ray autoradiographs of hindlimbs from the same animals shown in panel (A). Arrows point to the growth plate of tibia. The features typical of rickets shown in Hyp/Fgf23+/− (left panel) had improved considerably in compound mutants _Hyp/Fgf23_−/− (middle panel). Arrowheads depict thickness of femoral shaft of these animals. _Hyp/Fgf23_−/− compound mutants (middle) exhibit longer (brackets) and thinner long bones than Hyp/Fgf23+/− (left) animals.
Fig. 7
(A) Alizarin red S staining of skeletal elements (ribs, paws) from a Hyp/Fgf23+/− (left), _Hyp/Fgf23_−/− (middle), and _Fgf23_−/− (right) mice at 3 weeks. Arrows depict some areas with abnormal mineralization in _Hyp/Fgf23_−/− bones resembling the phenotype of _Fgf23_−/− skeleton. Lower panels represent 3μm-thick undecalcified sections from femur of 3-week-old Hyp/Fgf23+/− (left), _Hyp/Fgf23_−/− (middle), and _Fgf23_−/− (right) mice stained with Von Kossa/McNeal. Panel (B) represents a graph comparing serum phosphate levels of wild-type controls, _Fgf23_−/−, and _Hyp/Fgf23_−/− compound mutants at 3 weeks of age. The horizontal dotted line illustrates published serum phosphate levels in Hyp mice (mean: 4.45 mg/dl) (***=p<0.0001; **=p<0.001) (Lorenz-Depiereux et al., 2004).
Similar articles
- Overexpression of human PHEX under the human beta-actin promoter does not fully rescue the Hyp mouse phenotype.
Erben RG, Mayer D, Weber K, Jonsson K, Jüppner H, Lanske B. Erben RG, et al. J Bone Miner Res. 2005 Jul;20(7):1149-60. doi: 10.1359/JBMR.050212. Epub 2005 Feb 21. J Bone Miner Res. 2005. PMID: 15940367 - Distinct roles for intrinsic osteocyte abnormalities and systemic factors in regulation of FGF23 and bone mineralization in Hyp mice.
Liu S, Tang W, Zhou J, Vierthaler L, Quarles LD. Liu S, et al. Am J Physiol Endocrinol Metab. 2007 Dec;293(6):E1636-44. doi: 10.1152/ajpendo.00396.2007. Epub 2007 Sep 11. Am J Physiol Endocrinol Metab. 2007. PMID: 17848631 - FGF-23 inhibits renal tubular phosphate transport and is a PHEX substrate.
Bowe AE, Finnegan R, Jan de Beur SM, Cho J, Levine MA, Kumar R, Schiavi SC. Bowe AE, et al. Biochem Biophys Res Commun. 2001 Jun 22;284(4):977-81. doi: 10.1006/bbrc.2001.5084. Biochem Biophys Res Commun. 2001. PMID: 11409890 - FGF23, PHEX, and MEPE regulation of phosphate homeostasis and skeletal mineralization.
Quarles LD. Quarles LD. Am J Physiol Endocrinol Metab. 2003 Jul;285(1):E1-9. doi: 10.1152/ajpendo.00016.2003. Am J Physiol Endocrinol Metab. 2003. PMID: 12791601 Review. - Novel regulators of phosphate homeostasis and bone metabolism.
Jüppner H. Jüppner H. Ther Apher Dial. 2007 Oct;11 Suppl 1:S3-22. doi: 10.1111/j.1744-9987.2007.00513.x. Ther Apher Dial. 2007. PMID: 17976082 Review.
Cited by
- Osteocytic signalling pathways as therapeutic targets for bone fragility.
Plotkin LI, Bellido T. Plotkin LI, et al. Nat Rev Endocrinol. 2016 Oct;12(10):593-605. doi: 10.1038/nrendo.2016.71. Epub 2016 May 27. Nat Rev Endocrinol. 2016. PMID: 27230951 Free PMC article. Review. - FGF23 directly inhibits osteoprogenitor differentiation in Dmp1-knockout mice.
Courbon G, Kentrup D, Thomas JJ, Wang X, Tsai HH, Spindler J, Von Drasek J, Ndjonko LM, Martinez-Calle M, Lynch S, Hivert L, Wang X, Chang W, Feng JQ, David V, Martin A. Courbon G, et al. JCI Insight. 2023 Dec 22;8(24):e156850. doi: 10.1172/jci.insight.156850. JCI Insight. 2023. PMID: 37943605 Free PMC article. - Selective pharmacological inhibition of the sodium-dependent phosphate cotransporter NPT2a promotes phosphate excretion.
Clerin V, Saito H, Filipski KJ, Nguyen AH, Garren J, Kisucka J, Reyes M, Jüppner H. Clerin V, et al. J Clin Invest. 2020 Dec 1;130(12):6510-6522. doi: 10.1172/JCI135665. J Clin Invest. 2020. PMID: 32853180 Free PMC article. - Miscellaneous non-inflammatory musculoskeletal conditions. Hyperphosphatemic familial tumoral calcinosis (FGF23, GALNT3 and αKlotho).
Farrow EG, Imel EA, White KE. Farrow EG, et al. Best Pract Res Clin Rheumatol. 2011 Oct;25(5):735-47. doi: 10.1016/j.berh.2011.10.020. Best Pract Res Clin Rheumatol. 2011. PMID: 22142751 Free PMC article. Review. - FGF23 beyond mineral metabolism: a bridge to cardiovascular disease.
Larsson TE. Larsson TE. Clin J Am Soc Nephrol. 2011 Dec;6(12):2735-7. doi: 10.2215/CJN.10711011. Clin J Am Soc Nephrol. 2011. PMID: 22157705 Free PMC article. No abstract available.
References
- ADHR_Consortium Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. The ADHR Consortium. Nat. Genet. 2000;26:345–348. - PubMed
- Alfrey AC, LeGendre GR, Kaehny WD. The dialysis encephalopathy syndrome. Possible aluminum intoxication. N. Engl. J. Med. 1976;294:184–188. - PubMed
- Aono Y, Shimada T, Yamaziki Y, Hino R, Takeuchi Y, Fujita T, Fukumoto S, Nagano N, Wada M, Yamashita T. The neutralization of FGF-23 ameliorates hypophosphatemia and rickets in Hyp mice. J. Bone Miner. Res. 2003;18(Suppl. 2):1056.
- Argiro L, Desbarats M, Glorieux FH, Ecarot B. Mepe, the gene encoding a tumor-secreted protein in oncogenic hypophosphatemic osteomalacia, is expressed in bone. Genomics. 2001;74:342–351. - PubMed
- Bai X, Miao D, Panda D, Grady S, McKee MD, Goltzman D, Karaplis AC. Partial rescue of the Hyp phenotype by osteoblast-targeted PHEX (phosphate-regulating gene with homologies to endopeptidases on the X chromosome) expression. Mol. Endocrinol. 2002;16:2913–2925. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases