Dmp1-deficient mice display severe defects in cartilage formation responsible for a chondrodysplasia-like phenotype - PubMed (original) (raw)
. 2005 Feb 18;280(7):6197-203.
doi: 10.1074/jbc.M412911200. Epub 2004 Dec 7.
Yuji Mishina, Di Chen, Haiyang Huang, Sarah L Dallas, Mark R Dallas, Pitchumani Sivakumar, Tetsuo Kunieda, Takeo W Tsutsui, Adele Boskey, Lynda F Bonewald, Jian Q Feng
Affiliations
- PMID: 15590631
- PMCID: PMC2647591
- DOI: 10.1074/jbc.M412911200
Dmp1-deficient mice display severe defects in cartilage formation responsible for a chondrodysplasia-like phenotype
Ling Ye et al. J Biol Chem. 2005.
Abstract
Understanding the molecular mechanisms by which cartilage formation is regulated is essential toward understanding the physiology of both embryonic bone development and postnatal bone growth. Although much is known about growth factor signaling in cartilage formation, the regulatory role of noncollagenous matrix proteins in this process are still largely unknown. In the present studies, we present evidence for a critical role of DMP1 (dentin matrix protein 1) in postnatal chondrogenesis. The Dmp1 gene was originally identified from a rat incisor cDNA library and has been shown to play an important role in late stage dentinogenesis. Whereas no apparent abnormalities were observed in prenatal bone development, Dmp1-deficient (Dmp1(-/-)) mice unexpectedly develop a severe defect in cartilage formation during postnatal chondrogenesis. Vertebrae and long bones in Dmp1-deficient (Dmp1(-/-)) mice are shorter and wider with delayed and malformed secondary ossification centers and an irregular and highly expanded growth plate, results of both a highly expanded proliferation and a highly expanded hypertrophic zone creating a phenotype resembling dwarfism with chondrodysplasia. This phenotype appears to be due to increased cell proliferation in the proliferating zone and reduced apoptosis in the hypertrophic zone. In addition, blood vessel invasion is impaired in the epiphyses of Dmp1(-/-) mice. These findings show that DMP1 is essential for normal postnatal chondrogenesis and subsequent osteogenesis.
Figures
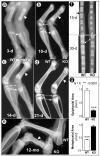
Fig. 1. Progressive changes of long bone structure in the _Dmp1_−/− mice
Representative radiographs of hind limbs from 3- (a), 10- (b), 14- (c), and 21-day-old (d) and 12-month-old animals (e). The shorter long bones with wider shafts of _Dmp1_−/− bones (KO) femurs are indicated by arrowheads, the delayed epiphyses are indicated by small arrows, and the expanded metaphyses are indicated by large arrows. Radiographs of tail vertebrae from 15- and 30-day-old mice show delayed formation of epiphyses and shortened vertebral bodies (f). Quantitative data for the extent of epiphyseal and metaphyseal ossification show a reduction in epiphysial area but an increase in metaphyseal area in _Dmp1_−/− mice (g).
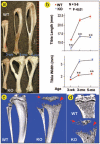
Fig. 2. Analyses of deformed tibias in _Dmp1_−/− mice
Photographs (a) and quantitative (b) analyses of tibias from 3-week-old and 3- and 5-month-old animals. A significant difference is observed between the WT and the mutant (KO) tibiae in both length and width. Representative three-dimensional micro-CT reconstruction of 1-month-old tibiae with whole mount view (c) and sagittal section view (d) are shown. The secondary ossification center is delayed and malformed (arrows), and the growth plate is dramatically expanded (arrowheads) in the tibia from _Dmp1_−/− mice.
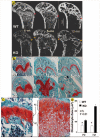
Fig. 3. Malformed growth plates in the _Dmp1_−/− mice
Images from backscattered scanning electron microscopy of 6-week-old and 6- and 12-month-old mice. Well formed growth plate (arrows), epiphyses and metaphyses can be easily seen in the WT mice (a, upper panel). The growth plate of _Dmp1_−/− mice however, is expanded and disorganized with malformed epiphyses and metaphyses (a, lower panel, arrows). Representative sections from decalcified femurs, stained with Safranin-O show a striking expansion of the growth plate at the age of 3 weeks and irregularities at 3 months and complete disruption at 5 months in the _Dmp1_−/− null mice (b, lower panel) compared with the WT growth plates (b, upper panel). Visually, histology shows an increased proliferation and hypertropic zone (c). Quantitative histomorphometry shows a significant increase in the proliferation zone of almost a doubling and in the hypertrophic zone of a 3-fold increase in femurs of 3-month-old _Dmp1_−/− mice compared with wild type controls (d). PZ, proliferation zone; HZ, hypertrophic zone.
Fig. 4. Increased proliferation, reduced apoptosis, and reduced calcification of the cartilaginous matrix and trabecular bone in the _Dmp1_−/− mice
A significant increase in cell proliferation as detected using bromodeoxyuridine labeling was observed in tibial growth plate of 2-month-old _Dmp1_−/− mice (a, arrows). No apparent difference in intensity of type II (b, arrowheads) and type X (c) collagen expression was observed in femora of 3-week-old _Dmp1_−/− mice. Caspase 3 staining showed a significant decrease in apoptosis of hypertrophic chondrocytes in 3-week-old _Dmp1_−/− mice (d). Goldner's Masson Trichrome staining of a tibial growth plate displays an expanded, disorganized growth plate, a poorly calcified cartilage matrix, and reduced trabecular bone formation in a 3-month-old _Dmp1_−/− mouse (e). PC, proliferating chondrocyte; HC, hypertrophic chondrocyte.
Fig. 5. Delayed blood vessel invasion in _Dmp1_−/− epiphyses
Polyclonal antibody against OPN (a), a marker of new bone formation is reduced in the KO epiphysis (arrow) but increased in the KO metaphysis (arrowhead) from a 3-week-old animal. Monoclonal antibody against PECAM-1 was used on sections of day 10 (b) and 17 (c) femurs. The positively stained endothelial cells (in blue purple, arrows) are significantly reduced in the _Dmp1_−/− epiphysis at day 10, although the stained endothelial cells in surrounding areas appear normal (arrowhead). By day 17, more endothelial cells stained positive for PECAM-1 in the _Dmp1_−/− epiphysis compared with the control with well formed bone marrow (c, arrow). In situ hybridization was performed to examine changes in mRNA expression of VEGF (d), and immunostaining was performed to examine protein expression of MMP9 (f), and enzyme activity of MMP9 (e) were measured by genetin zymography in 10-day-old _Dmp1_−/− mice and the age-matched control mice (right panels). Both VEGF and MMP9 are expressed at sites of neovascularization (arrows) with no apparent difference in VEGF and MMP9 expression in _Dmp1_−/− mice compared with control littermates. HC, hypertrophic chondrocyte.
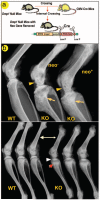
Fig. 6. Neither the mouse strain nor the neo cassette has an effect on the _Dmp1_−/− phenotype
To exclude the potential effect of the neo gene that was inserted into the Dmp1 knock-out construct on the bone phenotype of _Dmp1_−/− mice, CMV-cre transgenic mice were crossed with _Dmp1_−/− mice to remove the neo gene (a). The presence or absence of the neo gene has no effect on either the malformed epiphyses (arrowheads) or metaphysis (arrows) in 4-month-old _Dmp1_−/− mice (b, upper panel). Because the mouse strain itself can have considerable effect on bone structure and bone mineral density (35), we compared radiographs of Dmp1 null long bone of 1-month-old animals on the C57/B6 and the CD-1 background and found no apparent differences (b, lower panel).
Similar articles
- Roles of DMP1 processing in osteogenesis, dentinogenesis and chondrogenesis.
Sun Y, Chen L, Ma S, Zhou J, Zhang H, Feng JQ, Qin C. Sun Y, et al. Cells Tissues Organs. 2011;194(2-4):199-204. doi: 10.1159/000324672. Epub 2011 May 9. Cells Tissues Organs. 2011. PMID: 21555863 Free PMC article. - Cartilage-specific ablation of XBP1 signaling in mouse results in a chondrodysplasia characterized by reduced chondrocyte proliferation and delayed cartilage maturation and mineralization.
Cameron TL, Gresshoff IL, Bell KM, Piróg KA, Sampurno L, Hartley CL, Sanford EM, Wilson R, Ermann J, Boot-Handford RP, Glimcher LH, Briggs MD, Bateman JF. Cameron TL, et al. Osteoarthritis Cartilage. 2015 Apr;23(4):661-70. doi: 10.1016/j.joca.2015.01.001. Epub 2015 Jan 17. Osteoarthritis Cartilage. 2015. PMID: 25600960 - Dentin matrix protein 1, a target molecule for Cbfa1 in bone, is a unique bone marker gene.
Fen JQ, Zhang J, Dallas SL, Lu Y, Chen S, Tan X, Owen M, Harris SE, MacDougall M. Fen JQ, et al. J Bone Miner Res. 2002 Oct;17(10):1822-31. doi: 10.1359/jbmr.2002.17.10.1822. J Bone Miner Res. 2002. PMID: 12369786 - Regulatory mechanisms for the development of growth plate cartilage.
Michigami T. Michigami T. Cell Mol Life Sci. 2013 Nov;70(22):4213-21. doi: 10.1007/s00018-013-1346-9. Epub 2013 May 4. Cell Mol Life Sci. 2013. PMID: 23640571 Free PMC article. Review.
Cited by
- Growth deficiency in a mouse model of Kabuki syndrome 2 bears mechanistic similarities to Kabuki syndrome 1.
Gao CW, Lin W, Riddle RC, Chopra S, Kim J, Boukas L, Hansen KD, Björnsson HT, Fahrner JA. Gao CW, et al. PLoS Genet. 2024 Jun 10;20(6):e1011310. doi: 10.1371/journal.pgen.1011310. eCollection 2024 Jun. PLoS Genet. 2024. PMID: 38857303 Free PMC article. - Generation of bicistronic Dmp1-Cre knock-in mice using a self-cleaving 2A peptide.
Nakamura T, Honda S, Ito S, Mizoguchi T, Yamamoto T, Kasahara M, Kabe Y, Matsuo K, Suematsu M. Nakamura T, et al. J Bone Miner Metab. 2023 Jul;41(4):470-480. doi: 10.1007/s00774-023-01425-y. Epub 2023 Apr 10. J Bone Miner Metab. 2023. PMID: 37036533 - FGF4 and FGF9 have synergistic effects on odontoblast differentiation.
Hoshino T, Onodera S, Kimura M, Suematsu M, Ichinohe T, Azuma T. Hoshino T, et al. Med Mol Morphol. 2023 Sep;56(3):159-176. doi: 10.1007/s00795-023-00351-2. Epub 2023 Apr 3. Med Mol Morphol. 2023. PMID: 37012505 - The Emerging Role of Cell Transdifferentiation in Skeletal Development and Diseases.
Wang K, Ma C, Feng JQ, Jing Y. Wang K, et al. Int J Mol Sci. 2022 May 26;23(11):5974. doi: 10.3390/ijms23115974. Int J Mol Sci. 2022. PMID: 35682655 Free PMC article. Review. - Mechanical forces couple bone matrix mineralization with inhibition of angiogenesis to limit adolescent bone growth.
Dzamukova M, Brunner TM, Miotla-Zarebska J, Heinrich F, Brylka L, Mashreghi MF, Kusumbe A, Kühn R, Schinke T, Vincent TL, Löhning M. Dzamukova M, et al. Nat Commun. 2022 Jun 1;13(1):3059. doi: 10.1038/s41467-022-30618-8. Nat Commun. 2022. PMID: 35650194 Free PMC article.
References
- Kronenberg HM. Nature. 2003;423:332–336. - PubMed
- de Crombrugghe B, Lefebvre V, Nakashima K. Curr. Opin. Cell Biol. 2001;13:721–727. - PubMed
- Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Cell. 1997;89:747–754. - PubMed
- Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Cell. 1997;89:755–764. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AR051189-01/AR/NIAMS NIH HHS/United States
- R01 AR051189/AR/NIAMS NIH HHS/United States
- DE00455/DE/NIDCR NIH HHS/United States
- P01 AR046798/AR/NIAMS NIH HHS/United States
- AR46798/AR/NIAMS NIH HHS/United States
- R29 DE013480/DE/NIDCR NIH HHS/United States
- DE13480/DE/NIDCR NIH HHS/United States
- P30 AR046121/AR/NIAMS NIH HHS/United States
- R03 AR048920-02/AR/NIAMS NIH HHS/United States
- R03 AR048920/AR/NIAMS NIH HHS/United States
- K02 DE000455/DE/NIDCR NIH HHS/United States
- R01 AR051587/AR/NIAMS NIH HHS/United States
- AR51587/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials