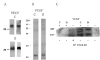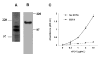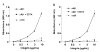The lymphangiogenic vascular endothelial growth factors VEGF-C and -D are ligands for the integrin alpha9beta1 - PubMed (original) (raw)
The lymphangiogenic vascular endothelial growth factors VEGF-C and -D are ligands for the integrin alpha9beta1
Nicholas E Vlahakis et al. J Biol Chem. 2005.
Abstract
Mice homozygous for a null mutation of the integrin alpha9 subunit die 6-12 days after birth from bilateral chylothoraces suggesting an underlying defect in lymphatic development. However, until now the mechanisms by which the integrin alpha9beta1 modulates lymphangiogenesis have not been described. In this study we show that adhesion to and migration on the lymphangiogenic vascular endothelial growth factors (VEGF-C and -D) are alpha9beta1-dependent. Mouse embryonic fibroblasts and human colon carcinoma cells (SW-480) transfected to express alpha9beta1 adhered and/or migrated on both growth factors in a concentration-dependent fashion, and both adhesion and migration were abrogated by anti-alpha9beta1 function-blocking antibody. In SW-480 cells, which lack cognate receptors for VEGF-C and -D, both growth factors induced alpha9beta1-dependent Erk and paxillin phosphorylation. Human microvascular endothelial cells, which express both alpha9beta1 and VEGF-R3, also adhered to and migrated on both growth factors, and both responses were blocked by anti-alpha9beta1 antibody. Furthermore, in a solid phase binding assay recombinant VEGF-C and -D bound to purified alpha9beta1 integrin in a dose- and cation-dependent fashion showing that VEGF-C and VEGF-D are ligands for the integrin alpha9beta1. The interaction between alpha9beta1 and VEGF-C and/or -D may begin to explain the abnormal lymphatic phenotype of the alpha9 knock-out mice.
Figures
FIG. 1. Production and purification of VEGF-C and D proteins
VEGF-C and D were purified from supernatant of transfected Drosphila S2 cells by Ni++ affinity chromatography. Proteins were separated by 15% SDS-PAGE under reducing conditions, and analyzed by (A) Western Blot analysis with V5 antibody (left panels) and VEGF-C and D specific antibodies (right panels), and (B) silver staining. (C) Phosphotyrosine blot from control HMVEC and HMVEC incubated with V5-tagged VEGFC or D. Lysates were immunoprecipitated with either no antibody or antibody against VEGFR-3, separated by 8% SDS-PAGE under reducing conditions and Western blotted with an anti-phosphotyrosine antibody. Molecular mass markers (kDa) are indicated to the left of all panels.
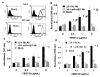
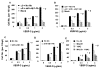
FIG. 2. α9-transfected MEF and SW-480 cells adhere to VEGF-C and D
(A) Flow cytometry analysis of mock (left) and α9 (right) transfected MEF (upper panel) and SW-480 cells (lower panel) used in cell adhesion and migration assays and for α9β1 purification (SW-480’s). For detection of α9β1 integrin, cells were labeled with the α9β1-specific antibody, Y9A2. Purified VEGF-C or D was used as substrate for adhesion assays with mock (diagonal bars) or α9 (black bars) transfected MEF (B & C) or SW-480’s (D) in the absence or presence (white bars) of the α9β1-blocking antibody, Y9A2. Cells were allowed to adhere to wells coated with a range of VEGF concentrations and then washed, fixed and stained with crystal violet. Adhesion is expressed as absorbance at 595 nm. * = p<0.05 compared to cells treated with Y9A2, # = p<0.05 compared to 0 ug/mL VEGF
FIG. 3. Binding of VEGF-C and D to α9β1 activates the integrin
Mock- or α9-transfected SW-480 cells were plated on various proteins for 15 min and cell lysates obtained. Following BCA protein quantification equal amounts of protein were separated under reducing conditions on 10% SDS-PAGE. (A) SW-480 cells (upper panel) plated for 15 min on 1% BSA, Tnfn3RAA (α9 specific ligand), VEGF-D or VEGF-C in the presence or absence of α9-blocking antibody (20 μg/mL) and immunoblotted with phospho-Erk 1/2 (upper panel). The PVDF membrane was re-probed with total Erk 2 to ensure equal protein loading (lower panel). (B) SW-480 cells plated for 15 min on VEGF-C or VEGF-D in the presence or absence of α9-blocking antibody (20 μg/mL) and immunoblotted with phosphopaxillin (upper panel). The PVDF membrane was re-probed with total paxillin to ensure equal protein loading (lower panel).
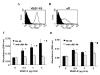
FIG. 4. Primary endothelial cell adhesion to VEGF-C and D is α9β1 dependent
Flow cytometry analysis of HMVEC labeled with 9D9f9 (A) and Y9A2 (B) to measure expression of VEGF-R3 and α9β1, respectively. Shaded areas represent cells labeled with no primary antibody and the unshaded area those labeled with the primary antibody, as indicated. Purified VEGF-C (C) and D (D) were used as substrate for adhesion assays with HMVEC in the absence (dark bars) or presence (white bars) of the α9β1-blocking antibody, Y9A2. Cells were processed as described in Fig. 2 above. * = p<0.05 compared to cells treated with Y9A2, # = p<0.05 compared to 0 ug/mL VEGF
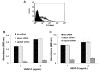
FIG. 5. siRNA silencing of α9 inhibits cell adhesion to VEGF-C and D
(A) Flow cytometry analysis of α9 expression in HMVEC, using the α9 specific antibody Y9A2, after no transfection, mock siRNA transfection (overlapping lines) or effective α9 siRNA transfection (shaded area). Unstained cells are also shown as a shaded area. VEGF-C (B) and D (C) were used as substrate for adhesion assays with α9 siRNA transfected (diagonal bars), mock transfected (white bars) or untransfected HMVEC (black bars).
FIG. 6. Cell migration on VEGF-C and D is α9β1-dependent
Migration assays on VEGF-C or D, coated on the lower surface of a Transwell at various concentrations, were performed with 1% fetal calf serum as a chemotactic factor. VEGF-C (A) or VEGF-D (B) was used as substrate for migration assays with mock (diagonal bars) or α9 (dark bars) transfected MEF in the absence or presence (white bars) of Y9A2. Similar assays were performed in HMVEC on VEGF-C (C) or VEGF-D (D) substrate in the absence (dark bars) or presence (white bars) of the α9β1-blocking antibody. (E) VEGF-D was used as substrate for migration assays with HMVEC in the absence (dark bars) or presence (white bars) of the α9β1-blocking antibody, Y9A2 and/or the VEGF-3 blocking drug, MAZ-51. * = p<0.05 compared to cells treated with Y9A2, # = p<0.05 compared to cells treated with MAZ-51 alone.
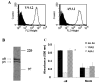
FIG. 7. A9A1, a specific non-blocking antibody to α9β1
(A) Flow cytometry analysis of α9 (line) and mock transfected (shaded area) MEF using the α9 antibody, A9A1 (right) compared to the α9β1 specific antibody, Y9A2 (left). (B) Autoradiograph of 35S labelled α9 transfected SW-480 cell lysates immunoprecipitated with 20μg of A9A1. The observed bands represent the α9 and β1 subunits of the integrin. (C) Tnfn3RAA, an α9-specific ligand, was used as substrate for adhesion assays in α9-transfected MEF in the absence (dark bars) or presence (diagonal bars) of A9A1 antibody and compared to the blocking antibody, Y9A2 (white bars). * = p<0.05 compared to cells treated with Y9A2
FIG. 8. Purification of active α9β1
α9β1 integrin was purified from cell lysates of α9-transfected SW-480 cells by affinity chromatography using an α9β1 antibody (A9A1) column. Proteins under reducing conditions were separated by 8% SDS-PAGE and analyzed by (A) silver staining and (B) Western Blot analysis with 1057, rabbit polyclonal antibody to α9β1. Molecular mass markers (kDa) are indicated. (C) Tnfn3RAA, an α9-specific ligand, was used as substrate for binding assays with purified α9β1 in the absence (diamonds) or presence (squares) of 10 mM EDTA.
FIG. 9. VEGF-C and D bind directly to the integrin α9β1
Purified VEGF-C (A) or D (B) was used for solid phase binding assays with purified α9β1 at various concentrations in the absence (diamonds) or presence (squares) of 10 mM EDTA. Similar assays were performed using purified αvβ6, an irrelevant integrin, (triangles).
Similar articles
- Co-expression of α9β1 integrin and VEGF-D confers lymphatic metastatic ability to a human breast cancer cell line MDA-MB-468LN.
Majumder M, Tutunea-Fatan E, Xin X, Rodriguez-Torres M, Torres-Garcia J, Wiebe R, Timoshenko AV, Bhattacharjee RN, Chambers AF, Lala PK. Majumder M, et al. PLoS One. 2012;7(4):e35094. doi: 10.1371/journal.pone.0035094. Epub 2012 Apr 24. PLoS One. 2012. PMID: 22545097 Free PMC article. - Integrin alpha9beta1 directly binds to vascular endothelial growth factor (VEGF)-A and contributes to VEGF-A-induced angiogenesis.
Vlahakis NE, Young BA, Atakilit A, Hawkridge AE, Issaka RB, Boudreau N, Sheppard D. Vlahakis NE, et al. J Biol Chem. 2007 May 18;282(20):15187-96. doi: 10.1074/jbc.M609323200. Epub 2007 Mar 14. J Biol Chem. 2007. PMID: 17363377 - Vascular endothelial growth factor regulates focal adhesion assembly in human brain microvascular endothelial cells through activation of the focal adhesion kinase and related adhesion focal tyrosine kinase.
Avraham HK, Lee TH, Koh Y, Kim TA, Jiang S, Sussman M, Samarel AM, Avraham S. Avraham HK, et al. J Biol Chem. 2003 Sep 19;278(38):36661-8. doi: 10.1074/jbc.M301253200. Epub 2003 Jul 3. J Biol Chem. 2003. PMID: 12844492 - Molecular mechanisms of lymphangiogenesis.
Takahashi M, Yoshimoto T, Kubo H. Takahashi M, et al. Int J Hematol. 2004 Jul;80(1):29-34. doi: 10.1532/ijh97.04042. Int J Hematol. 2004. PMID: 15293565 Review. - Lymphangiogenic growth factors as markers of tumor metastasis.
Stacker SA, Williams RA, Achen MG. Stacker SA, et al. APMIS. 2004 Jul-Aug;112(7-8):539-49. doi: 10.1111/j.1600-0463.2004.apm11207-0812.x. APMIS. 2004. PMID: 15563315 Review.
Cited by
- Interaction between the extracellular matrix and lymphatics: consequences for lymphangiogenesis and lymphatic function.
Wiig H, Keskin D, Kalluri R. Wiig H, et al. Matrix Biol. 2010 Oct;29(8):645-56. doi: 10.1016/j.matbio.2010.08.001. Epub 2010 Aug 18. Matrix Biol. 2010. PMID: 20727409 Free PMC article. Review. - Visualization of integrin molecules by fluorescence imaging and techniques.
Cai C, Sun H, Hu L, Fan Z. Cai C, et al. Biocell. 2021;45(2):229-257. doi: 10.32604/biocell.2021.014338. Epub 2021 Feb 19. Biocell. 2021. PMID: 34219865 Free PMC article. - Understanding lymphangiogenesis in knockout models, the cornea, and ocular diseases for the development of therapeutic interventions.
Yang JF, Walia A, Huang YH, Han KY, Rosenblatt MI, Azar DT, Chang JH. Yang JF, et al. Surv Ophthalmol. 2016 May-Jun;61(3):272-96. doi: 10.1016/j.survophthal.2015.12.004. Epub 2015 Dec 17. Surv Ophthalmol. 2016. PMID: 26706194 Free PMC article. Review. - Traumatic brain injury in mice induces changes in the expression of the XCL1/XCR1 and XCL1/ITGA9 axes.
Ciechanowska A, Popiolek-Barczyk K, Ciapała K, Pawlik K, Oggioni M, Mercurio D, de Simoni MG, Mika J. Ciechanowska A, et al. Pharmacol Rep. 2020 Dec;72(6):1579-1592. doi: 10.1007/s43440-020-00187-y. Epub 2020 Nov 13. Pharmacol Rep. 2020. PMID: 33185818 Free PMC article. - Very late antigen 1 blockade markedly promotes survival of corneal allografts.
Chen L, Huq S, Gardner H, de Fougerolles AR, Barabino S, Dana MR. Chen L, et al. Arch Ophthalmol. 2007 Jun;125(6):783-8. doi: 10.1001/archopht.125.6.783. Arch Ophthalmol. 2007. PMID: 17562989 Free PMC article.
References
- Hynes RO. Cell. 1992;69:11–25. - PubMed
- Friedl P. Curr Opin Cell Biol. 2004;16:14–23. - PubMed
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Science. 2003;302:1704–1709. - PubMed
- Hynes RO. Cell. 2002;110:673–687. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous