The unfolding of the P pili quaternary structure by stretching is reversible, not plastic - PubMed (original) (raw)
The unfolding of the P pili quaternary structure by stretching is reversible, not plastic
Erik Fällman et al. EMBO Rep. 2005 Jan.
Abstract
P pili are protein filaments expressed by uropathogenic Escherichia coli that mediate binding to glycolipids on epithelial cell surfaces, which is a prerequisite for bacterial infection. When a bacterium, attached to a cell surface, is exposed to external forces, the pili, which are composed of approximately 10(3) PapA protein subunits arranged in a helical conformation, can elongate by unfolding to a linear conformation. This property is considered important for the ability of a bacterium to withstand shear forces caused by urine flow. It has hitherto been assumed that this elongation is plastic, thus constituting a permanent conformational deformation. We demonstrate, using optical tweezers, that this is not the case; the unfolding of the helical structure to a linear conformation is fully reversible. It is surmised that this reversibility helps the bacteria regain close contact to the host cells after exposure to significant shear forces, which is believed to facilitate their colonization.
Figures
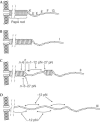
Figure 1
Schematic illustration of a P pilus in its unstretched native form (A) as well as in three different elongation regions (I–III; B–D). A P pilus consists of a PapA rod (6.8 nm in diameter) arranged in a helix (Gong & Makowski, 1992) that is anchored to a bacterial membrane by PapH (Båga et al, 1987) and connected to a fibrillum by a PapK adaptor (Jacob-Dubuisson et al, 1993). The fibrillum tip (15 nm long and 2–3 nm in diameter; Bullitt & Makowski, 1998) is a flexible structure composed of PapE (Lindberg et al, 1986), PapF (Lindberg et al, 1986) and the PapG (Lindberg et al, 1987; Kuehn et al, 1992; Jacob-Dubuisson et al, 1993) adhesin. The forces from the adjacent-turn and the head-to-tail interactions are indicated.
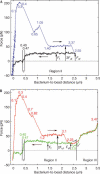
Figure 2
Elongation and contraction study of P pili. Two consecutive elongation and contraction cycles are shown, displayed by the blue and black curves in (A) and by the red and green curves in (B), respectively. The numbers in the graphs indicate important bacterium-to-bead distances. The regions of the remaining pilus during contraction are indicated (I, II or III). The unfolding and folding forces are indicated with arrows.
Similar articles
- Dynamic force spectroscopy of E. coli P pili.
Andersson M, Fällman E, Uhlin BE, Axner O. Andersson M, et al. Biophys J. 2006 Oct 1;91(7):2717-25. doi: 10.1529/biophysj.106.087429. Epub 2006 Jul 14. Biophys J. 2006. PMID: 16844748 Free PMC article. - Fast uncoiling kinetics of F1C pili expressed by uropathogenic Escherichia coli are revealed on a single pilus level using force-measuring optical tweezers.
Castelain M, Ehlers S, Klinth J, Lindberg S, Andersson M, Uhlin BE, Axner O. Castelain M, et al. Eur Biophys J. 2011 Mar;40(3):305-16. doi: 10.1007/s00249-010-0648-1. Epub 2010 Dec 16. Eur Biophys J. 2011. PMID: 21161524 - Structural and energetic basis of folded-protein transport by the FimD usher.
Geibel S, Procko E, Hultgren SJ, Baker D, Waksman G. Geibel S, et al. Nature. 2013 Apr 11;496(7444):243-6. doi: 10.1038/nature12007. Nature. 2013. PMID: 23579681 Free PMC article. - Structural and functional insights into the assembly of type 1 pili from Escherichia coli.
Capitani G, Eidam O, Glockshuber R, Grütter MG. Capitani G, et al. Microbes Infect. 2006 Jul;8(8):2284-90. doi: 10.1016/j.micinf.2006.03.013. Epub 2006 Jun 2. Microbes Infect. 2006. PMID: 16793308 Review. - Physical properties of biopolymers assessed by optical tweezers: analysis of folding and refolding of bacterial pili.
Andersson M, Axner O, Almqvist F, Uhlin BE, Fällman E. Andersson M, et al. Chemphyschem. 2008 Feb 1;9(2):221-35. doi: 10.1002/cphc.200700389. Chemphyschem. 2008. PMID: 18181116 Review.
Cited by
- Cells as liquid motors: mechanosensitivity emerges from collective dynamics of actomyosin cortex.
Étienne J, Fouchard J, Mitrossilis D, Bufi N, Durand-Smet P, Asnacios A. Étienne J, et al. Proc Natl Acad Sci U S A. 2015 Mar 3;112(9):2740-5. doi: 10.1073/pnas.1417113112. Epub 2015 Feb 17. Proc Natl Acad Sci U S A. 2015. PMID: 25730854 Free PMC article. - Surface organelles assembled by secretion systems of Gram-negative bacteria: diversity in structure and function.
Thanassi DG, Bliska JB, Christie PJ. Thanassi DG, et al. FEMS Microbiol Rev. 2012 Nov;36(6):1046-82. doi: 10.1111/j.1574-6976.2012.00342.x. Epub 2012 May 24. FEMS Microbiol Rev. 2012. PMID: 22545799 Free PMC article. Review. - Uncoiling mechanics of Escherichia coli type I fimbriae are optimized for catch bonds.
Forero M, Yakovenko O, Sokurenko EV, Thomas WE, Vogel V. Forero M, et al. PLoS Biol. 2006 Sep;4(9):e298. doi: 10.1371/journal.pbio.0040298. PLoS Biol. 2006. PMID: 16933977 Free PMC article. - Rigid multibody simulation of a helix-like structure: the dynamics of bacterial adhesion pili.
Zakrisson J, Wiklund K, Servin M, Axner O, Lacoursière C, Andersson M. Zakrisson J, et al. Eur Biophys J. 2015 Jul;44(5):291-300. doi: 10.1007/s00249-015-1021-1. Epub 2015 Apr 8. Eur Biophys J. 2015. PMID: 25851543 - The assembly platform FimD is required to obtain the most stable quaternary structure of type 1 pili.
Zyla DS, Wiegand T, Bachmann P, Zdanowicz R, Giese C, Meier BH, Waksman G, Hospenthal MK, Glockshuber R. Zyla DS, et al. Nat Commun. 2024 Apr 8;15(1):3032. doi: 10.1038/s41467-024-47212-9. Nat Commun. 2024. PMID: 38589417 Free PMC article.
References
- Båga M, Norgren M, Normark S (1987) Biogenesis of Escherichia coli Pap pili—PapH, a minor pilin subunit involved in cell anchoring and length modulation. Cell 49: 241–251 - PubMed
- Bullitt E, Makowski L (1995) Structural polymorphism of bacterial adhesion pili. Nature 373: 164–167 - PubMed
- Bullitt E, Gong M, Makowski L (1992) A molecular bungee cord—3-dimensional helical reconstruction of bacterial adhesion pili by electron-microscopy. Mol Biol Cell 3: A171
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources