Caspases and nitric oxide broadly regulate dendritic cell maturation and surface expression of class II MHC proteins - PubMed (original) (raw)
Caspases and nitric oxide broadly regulate dendritic cell maturation and surface expression of class II MHC proteins
Siew Heng Wong et al. Proc Natl Acad Sci U S A. 2004.
Abstract
The passage of dendritic cells (DC) from immature to terminally differentiated antigen-presenting cells is accompanied by numerous morphological, phenotypic, and functional changes. These changes include, for example, expression of "empty" class II MHC proteins (MHCII) at the surface in immature DC, whereas a much larger amount of peptide-loaded MHCII is expressed at the surface in mature DC. Here we show that, in cultured immature DC derived from murine bone-marrow precursors, a number of molecules involved in intracellular trafficking were present in a cleaved form, degraded by caspase-like proteases. Cleavage was either inhibited or reduced significantly during maturation of DC induced by either LPS and TNF-alpha or by peptides that inhibit caspase activities. Inducible nitric oxide (NO) synthetase up-regulated by LPS was essential for inhibiting the caspase-like activity during the maturation of DC. Moreover, treatment with LPS or caspase inhibitor resulted in expression of MHCII/peptide complexes at the cell surface. Thus, the alteration of the endosomal trafficking pathways during the development of DC that parallels the changes in surface expression of MHCII is regulated at least in part by the activities of caspases, inducible NO synthetase, and its product NO.
Figures
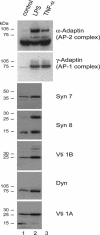
Fig. 1.
Expression of essential molecules of the endosomal pathways during DC maturation. Untreated (control) and LPS- or TNF-α-treated DC were extracted in buffer containing 2% Triton X-100 for 1 h on ice. Extracts (50 μg) were separated by 12% mini-SDS/PAGE and analyzed by Western blot by using Abs against α-adaptin, γ-adaptin, syntaxin (Syn) 7, syntaxin 8, dynamin (Dyn), Vti1A, and Vti1B. All of the Abs used in this experiment were mouse mAb, except for anti-syntaxin 7, which was a polyclonal Ab raised in a rabbit.
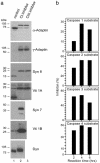
Fig. 2.
Effects of caspase inhibitors. (a) Inhibition of protein cleavage of endosomal trafficking molecules. DC were either treated with DMSO (control), group I caspase (CI) inhibitor, or group III caspase (CIII) inhibitor for 4 h before lysis and treatment with extraction buffer for 1 h on ice. Extracts (50 μg) were separated by 12% mini-SDS/PAGE and analyzed by Western blot by using Abs against α-adaptin, γ-adaptin, Vti1A, Vti1B, syntaxin (Syn) 7, syntaxin 8, and dynamin (Dyn) as probes. For Vti1B, syntaxin 7, and dynamin, only the DMSO- and group I caspase inhibitor-treated DC were analyzed. (b) Inhibition by LPS of cleavage of substrates for specific caspases. Extracts (20 μg) from untreated and LPS-treated DC were incubated with fluorescence-labeled substrates for caspase-1, caspase-2, caspase-3, and caspase-4 (see Materials and Methods) for 2, 4, and 6 h. The inhibition of caspase activity during DC maturation induced by LPS treatment is shown.
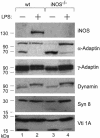
Fig. 4.
Caspase cleavage activities increased in iNOS-deficient DC. DC prepared from wild-type (wt) and iNOS-deficient (iNOS-/-) mice were either untreated or treated with LPS for 40 h before protein extract preparations. Protein extracts were analyzed by Western blot by using mAb against iNOS, α-adaptin, γ-adaptin, dynamin, syntaxin (Syn) 8, and Vti1A.
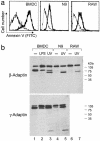
Fig. 6.
β- and γ-adaptin cleavage in maturing DC is different from that occurring during apoptosis. (a) BMDC, N9, and RAW cells were UV irradiated for 2 min to induce apoptosis. After 6 h of culture, a fraction of control or irradiated cells was stained with annexin V-FITC to ensure the irradiated population was in apoptosis. (b) Total cell lysate was prepared, and β- and γ-adaptin were analyzed by Western blot. As previously observed, only untreated BMDC present cleaved adaptin fragments. After UV irradiation, β- and γ-adaptin were cleaved in all cell types. The size of the cleaved fragment was different from the one previously observed in untreated BMDC, suggesting that different caspase pathways are active during DC maturation or during the apoptotic processes.
Fig. 3.
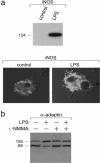
iNOS, induced by LPS, reduced the cleavage of α-adaptin and γ-adaptin during DC maturation. (a Upper) Protein extracts (80 μg) from immature (untreated) and mature DC (plus LPS) were analyzed by Western blot by employing mAb specific for iNOS (NOS2). (a Lower) Confocal microscopic analysis for iNOS (using anti-iNOS phycoerythrin) performed on immature and LPS-matured DC as above. The red dye appears white in this black and white figure. (b) Extracts from DC either untreated (lane 1) or treated with the iNOS inhibitor
l
-NMMA (lane 2), LPS (lane 3), or
l
-NMMA plus LPS (lane 4) were analyzed by Western blot by using Abs against α-adaptin.
Fig. 5.
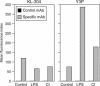
DC treatment with group I caspase inhibitor (CI) as well as LPS induces MHCII redistribution to the cell surface. Cell-surface staining of GM-CSF, SJL/J BMDC untreated or treated with LPS or group I caspase inhibitor with the mAbs specific for empty (KL-304) or peptide-loaded (Y3P) MHCII. A typical experiment is shown.
Similar articles
- Nitric-oxide synthase 2 interacts with CD74 and inhibits its cleavage by caspase during dendritic cell development.
Huang D, Cai DT, Chua RYR, Kemeny DM, Wong SH. Huang D, et al. J Biol Chem. 2008 Jan 18;283(3):1713-1722. doi: 10.1074/jbc.M705998200. Epub 2007 Nov 14. J Biol Chem. 2008. PMID: 18003616 - Differential susceptibility to CD95 (Apo-1/Fas) and MHC class II-induced apoptosis during murine dendritic cell development.
McLellan AD, Terbeck G, Mengling T, Starling GC, Kiener PA, Gold R, Bröcker EB, Leverkus M, Kämpgen E. McLellan AD, et al. Cell Death Differ. 2000 Oct;7(10):933-8. doi: 10.1038/sj.cdd.4400734. Cell Death Differ. 2000. PMID: 11279539 - Involvement of caspase-cleaved and intact adaptor protein 1 complex in endosomal remodeling in maturing dendritic cells.
Santambrogio L, Potolicchio I, Fessler SP, Wong SH, Raposo G, Strominger JL. Santambrogio L, et al. Nat Immunol. 2005 Oct;6(10):1020-8. doi: 10.1038/ni1250. Epub 2005 Sep 18. Nat Immunol. 2005. PMID: 16170319 - Control of MHC class II antigen presentation in dendritic cells: a balance between creative and destructive forces.
Villadangos JA, Schnorrer P, Wilson NS. Villadangos JA, et al. Immunol Rev. 2005 Oct;207:191-205. doi: 10.1111/j.0105-2896.2005.00317.x. Immunol Rev. 2005. PMID: 16181337 Review. - Regulation of caspases by nitric oxide.
Kim PK, Kwon YG, Chung HT, Kim YM. Kim PK, et al. Ann N Y Acad Sci. 2002 May;962:42-52. doi: 10.1111/j.1749-6632.2002.tb04054.x. Ann N Y Acad Sci. 2002. PMID: 12076961 Review.
Cited by
- Caspases as therapeutic targets.
Howley B, Fearnhead HO. Howley B, et al. J Cell Mol Med. 2008 Sep-Oct;12(5A):1502-16. doi: 10.1111/j.1582-4934.2008.00292.x. Epub 2008 Feb 24. J Cell Mol Med. 2008. PMID: 18298652 Free PMC article. Review. - Nitric oxide and cGMP protein kinase (cGK) regulate dendritic-cell migration toward the lymph-node-directing chemokine CCL19.
Giordano D, Magaletti DM, Clark EA. Giordano D, et al. Blood. 2006 Feb 15;107(4):1537-45. doi: 10.1182/blood-2005-07-2901. Epub 2005 Oct 25. Blood. 2006. PMID: 16249377 Free PMC article. - AP-1 and ARF1 control endosomal dynamics at sites of FcR mediated phagocytosis.
Braun V, Deschamps C, Raposo G, Benaroch P, Benmerah A, Chavrier P, Niedergang F. Braun V, et al. Mol Biol Cell. 2007 Dec;18(12):4921-31. doi: 10.1091/mbc.e07-04-0392. Epub 2007 Oct 3. Mol Biol Cell. 2007. PMID: 17914058 Free PMC article. - Physiological functions of caspases beyond cell death.
Nhan TQ, Liles WC, Schwartz SM. Nhan TQ, et al. Am J Pathol. 2006 Sep;169(3):729-37. doi: 10.2353/ajpath.2006.060105. Am J Pathol. 2006. PMID: 16936249 Free PMC article. Review. No abstract available. - Apoptosis-induced inhibition of CD1d-mediated antigen presentation: different roles for caspases and signal transduction pathways.
Khan MA, Sriram V, Renukaradhya GJ, Du W, Gervay-Hague J, Brutkiewicz RR. Khan MA, et al. Immunology. 2008 Sep;125(1):80-90. doi: 10.1111/j.1365-2567.2008.02823.x. Epub 2008 Mar 14. Immunology. 2008. PMID: 18346153 Free PMC article.
References
- Banchereau, J., Briere, F., Caux, C., Davoust, J., Lebecque, S., Liu, Y. J., Pulendran, B. & Palucka, K. I. (2000) Annu. Rev. Immunol. 18, 767-811. - PubMed
- Banchereau, J. & Steinman, R. M. (1998) Nature 392, 245-252. - PubMed
- Lanzavecchia, A. & Sallusto, F. (2001) Cell 106, 263-266. - PubMed
- Garrett, W. S., Chen, L., Kroschewski, R., Ebersold, M., Turley, S., Trombetta, S., Galan, J. E. & Mellman, I. (2000) Cell 102, 325-334. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI049524/AI/NIAID NIH HHS/United States
- R01 AI048832/AI/NIAID NIH HHS/United States
- CA47554/CA/NCI NIH HHS/United States
- AI-48832/AI/NIAID NIH HHS/United States
- AI-49524/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials