VHL-box and SOCS-box domains determine binding specificity for Cul2-Rbx1 and Cul5-Rbx2 modules of ubiquitin ligases - PubMed (original) (raw)
Comparative Study
. 2004 Dec 15;18(24):3055-65.
doi: 10.1101/gad.1252404.
Affiliations
- PMID: 15601820
- PMCID: PMC535916
- DOI: 10.1101/gad.1252404
Comparative Study
VHL-box and SOCS-box domains determine binding specificity for Cul2-Rbx1 and Cul5-Rbx2 modules of ubiquitin ligases
Takumi Kamura et al. Genes Dev. 2004.
Abstract
The ECS (Elongin B/C-Cul2/Cul5-SOCS-box protein) complex is a member of a family of ubiquitin ligases that share a Cullin-Rbx module. SOCS-box proteins recruit substrates to the ECS complex and are linked to Cullin-Rbx via Elongin B/C. VHL has been implicated as a SOCS-box protein, but lacks a C-terminal sequence (downstream of the BC box) of the SOCS box. We now show that VHL specifically interacts with endogenous Cul2-Rbx1 in mammalian cells, whereas SOCS-box proteins associate with Cul5-Rbx2. We also identify LRR-1 and FEM1B as proteins that share a region of homology with VHL (the VHL box, including the BC box and downstream residues) and associate with Cul2-Rbx1. ECS complexes can thus be classified into two distinct protein assemblies, that is, those that contain a subunit with a VHL box (composed of the BC box and a downstream Cul2 box) that interacts with Cul2-Rbx1, and those that contain a subunit with a SOCS box (BC box and downstream Cul5 box) that interacts with Cul5-Rbx2. Domain-swapping analyses showed that the specificity of interaction of VHL-box and SOCS-box proteins with Cullin-Rbx modules is determined by the Cul2 and Cul5 boxes, respectively. Finally, RNAi-mediated knockdown of the Cul2-Rbx1 inhibited the VHL-mediated degradation of HIF-2alpha, whereas knockdown of Cul5-Rbx2 did not affect it. These data suggest that the functions of the Cul2-Rbx1 and Cul5-Rbx2 modules are distinct.
Figures
Figure 6.
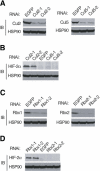
Effect of RNAi knockdown of cullin-Rbx modules on the HIF-2α expression. (A,B) HeLa cells were infected with with a retroviral vector encoding either Cul2 (Cul2-1 or Cul2-2), Cul5 (Cul5-1 or Cul5-2), or EGFP (control) shRNAs. Lysates of the cells were subjected to immunoblot analysis with anti-Cul2, anti-Cul5, anti-HIF-2α, or anti-HSP90 (control). (C,D) Knockdown experiments for Rbx1 (Rbx1-1 or Rbx1-2) or Rbx2 (Rbx2-1 or Rbx2-2) were performed as in A and B.
Figure 1.
Sequence-based classification of VHL-box and SOCS-box proteins. (A) Domain organization of VHL-box and SOCS-box proteins. (B) Alignment of amino acid sequences of VHL-box and SOCS-box proteins. The BC box, Cul2 box, and Cul5 box are indicated (boxed). Identical (black) and similar (gray) amino acids are shaded. Asterisks indicate C termini. Sequences are of the human proteins with the exception of SOCS1, WSB1, and MUF1.
Figure 2.
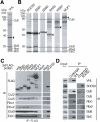
Identification of proteins that associate with VHL or SOCS-box proteins. (A,B) Lysates of HEK293T cells stably expressing the 3xFlag-tagged recombinant proteins indicated at the top of each lane were subjected to immunoprecipitation with anti-Flag, and the resulting precipitates were fractionated by SDS-PAGE and stained with Coomassie blue. Proteins identified by mass spectrometric analysis are indicated, as are the 3xFlag-tagged proteins (arrowheads). (EloB) Elongin B; (EloC) Elongin C. (C) Immunoprecipitates (IP) similar to those obtained in A were subjected to immunoblot analysis (IB) with antibodies to Flag or to the indicated proteins. Mock indicates cells transfected with empty vector. (D) RAW264.7 cells were incubated for 5 h with lipopolysaccharide (10 ng/mL-1) and then for 5 h with 10 μM MG132, after which cell lysates were subjected to immunoprecipitation with either anti-VHL, anti-SOCS3, or control IgG. The cell extracts (1% of the input for immunoprecipitation) and the resulting precipitates were subjected to immunoblot analysis with antibodies to the indicated proteins.
Figure 3.
The Cul5 box of SOCS-box proteins is required for interaction with the Cul5-Rbx2 module. (A) Domain organization of human wild-type RAR3 (RAR3-WT) and the M1, M2, and M3 mutants thereof. BC-box (filled) and Cul5-box (dotted) are shown. (B) Lysates of HEK293T cells stably expressing the indicated 3xFlag-tagged RAR3 derivatives were subjected to immunoprecipitation with anti-Flag, and the resulting precipitates were subjected to immunoblot analysis with antibodies to the indicated proteins. (C) Domain organization of human wild-type SSB1 (SSB1-WT) and the M1 and M2 mutants thereof. (D) Lysates of HEK293T cells stably expressing the indicated 3xFlag-tagged SSB1 derivatives were subjected to immunoprecipitation with anti-Flag, and the resulting precipitates were subjected to immunoblot analysis with antibodies to the indicated proteins. (E) Domain organization of mouse wild-type SOCS1 (SOCS1-WT) and the M1, M2, and M3 mutants thereof. (F,G) Lysates of HEK293T cells stably expressing the indicated 3xFlag-tagged SOCS1 derivatives were subjected to immunoprecipitation with anti-Flag, and the resulting precipitates were analyzed by SDS-PAGE and mass spectrometry (F) or by immunoblotting (G) as described in Figure 2.
Figure 4.
Chimeric proteins composed of VHL and a Cul5 box assemble with the Cul5-Rbx2 module. (A) Domain organization of human wild-type VHL (VHL-WT) and the M1, M2, and M3 derivatives thereof. BC-box (filled), Cul2-box (hatched), and Cul5-box (dotted) are shown. (B) Lysates of 786O cells stably expressing the indicated 3xFlag-tagged VHL derivatives were subjected to immunoprecipitation with anti-Flag, and the resulting precipitates were subjected to immunoblot analysis with antibodies to the indicated proteins. (C) Lysates of 786O cells stably expressing the indicated 3xFlag-tagged VHL derivatives were subjected to immunoblot analysis with antibodies to Flag, to HIF-2α, or to HSP90 (loading control). (D) Total RNA isolated from 786O cells stably expressing the indicated 3xFlag-tagged VHL derivatives was subjected to Northern blot analysis with a VEGF-specific probe. Ethidium bromide staining of 28S rRNA is also shown (loading control).
Figure 5.
Identification of LRR-1 and FEM1B as VHL-box proteins that interact specifically with endogenous Cul2-Rbx1. (A-C) Lysates of HEK293T cells transiently (A) or stably (B,C) expressing the indicated recombinant proteins were subjected to immunoprecipitation with anti-Flag, and the resulting precipitates were analyzed by SDS-PAGE and mass spectrometry (A,B) and immunoblotting (C) as described in Figure 2. (D) Domain organization of human wild-type VHL (VHL-WT) and the M4 mutant thereof, as well as of human wild-type LRR-1 (LRR-1-WT), and the M1 mutant thereof. BC-box (filled) and Cul2-box (hatched) are shown. (E,F) Lysates of HEK293T cells stably expressing the indicated recombinant proteins were subjected to immunoprecipitation with anti-Flag, and the resulting precipitates were analyzed by SDS-PAGE and mass spectrometry (E) and immunoblotting (F) as described in Figure 2. (G) Domain organization of human wild-type SSB4 (SSB4-WT) and the M1 mutant thereof. BC-box (filled) and Cul5-box (dotted) are shown. (H) Lysates of HEK293T cells stably expressing the indicated recombinant proteins were subjected to immunoprecipitation with anti-Flag, and the resulting precipitates were subjected to immunoblot analysis with antibodies to the indicated proteins.
Figure 7.
Structural comparison of putative Cullin-binding interfaces. (A) Comparison of the SCFSkp2 complex (left) and the VHL-Elongin B/C complex (right). Ribbon models of Cul1 (residues 17-355), Skp1, and Skp2 are shown in gray, green, and purple, respectively. Ribbon models of VHL (residues 62-209), Elongin B, and Elongin C are shown in magenta, orange, and red, respectively. Superposition of the VHL-Elongin B/C complex on the SCFSkp2 complex is also shown (middle) in the view of the similar structural motifs of Elongin C and Skp1. The F-box region of Skp2 directly binds to the N-terminal region of Cul1 (orange dotted circle). (B) The Cul1-binding interface of Skp2 and the putative corresponding site (Cul2 or VHL box) of VHL, RAR3 and SOCS1, each of which is highlighted by the orange dotted circle in A, are shown as ball-and-stick models. The red circle shown for the SCFSkp2 complex corresponds to the LPXP motif in the SOCS box. Color coding of proteins is the same as that in A. (C) Schematic representation of the amino acids at the Cullin-binding interfaces of Skp2, VHL, RAR3, and SOCS1. Hydrophilic, hydrophobic, and charged residues are shown in green, gray, and blue (positive) or red (negative), respectively. Threonine and tyrosine residues have both hydrophobic regions and hydrophilic hydroxyl groups, and are thus shown in gray and green (OH). Asp 198 of SOCS1 appears unable to participate in a hydrophobic interaction (cross). (D) Amino acid sequence comparisons among Cul1, Cul2, and Cul5 in the putative F-box, VHL-box, and SOCS-box-binding sites, respectively (left), as well as among Skp2, VHL, SOCS1, and RAR3 in the putative Cullin-binding sites. The LPXP motif of the SOCS box is underlined. Boxed residues are thought to participate in interactions. All residue numbers correspond to the human proteins.
Similar articles
- Characterization of Cullin-box sequences that direct recruitment of Cul2-Rbx1 and Cul5-Rbx2 modules to Elongin BC-based ubiquitin ligases.
Mahrour N, Redwine WB, Florens L, Swanson SK, Martin-Brown S, Bradford WD, Staehling-Hampton K, Washburn MP, Conaway RC, Conaway JW. Mahrour N, et al. J Biol Chem. 2008 Mar 21;283(12):8005-13. doi: 10.1074/jbc.M706987200. Epub 2008 Jan 10. J Biol Chem. 2008. PMID: 18187417 - ASB proteins interact with Cullin5 and Rbx2 to form E3 ubiquitin ligase complexes.
Kohroki J, Nishiyama T, Nakamura T, Masuho Y. Kohroki J, et al. FEBS Lett. 2005 Dec 19;579(30):6796-802. doi: 10.1016/j.febslet.2005.11.016. Epub 2005 Nov 28. FEBS Lett. 2005. PMID: 16325183 - Muf1, a novel Elongin BC-interacting leucine-rich repeat protein that can assemble with Cul5 and Rbx1 to reconstitute a ubiquitin ligase.
Kamura T, Burian D, Yan Q, Schmidt SL, Lane WS, Querido E, Branton PE, Shilatifard A, Conaway RC, Conaway JW. Kamura T, et al. J Biol Chem. 2001 Aug 10;276(32):29748-53. doi: 10.1074/jbc.M103093200. Epub 2001 May 30. J Biol Chem. 2001. PMID: 11384984 - Role of SOCS and VHL Proteins in Neuronal Differentiation and Development.
Kanno H, Matsumoto S, Yoshizumi T, Nakahara K, Kubo A, Murata H, Shuin T, U HS. Kanno H, et al. Int J Mol Sci. 2023 Feb 15;24(4):3880. doi: 10.3390/ijms24043880. Int J Mol Sci. 2023. PMID: 36835292 Free PMC article. Review. - The role of cullin 5-containing ubiquitin ligases.
Okumura F, Joo-Okumura A, Nakatsukasa K, Kamura T. Okumura F, et al. Cell Div. 2016 Mar 9;11:1. doi: 10.1186/s13008-016-0016-3. eCollection 2016. Cell Div. 2016. PMID: 27030794 Free PMC article. Review.
Cited by
- SOCS proteins in infectious diseases of mammals.
Delgado-Ortega M, Marc D, Dupont J, Trapp S, Berri M, Meurens F. Delgado-Ortega M, et al. Vet Immunol Immunopathol. 2013 Jan 15;151(1-2):1-19. doi: 10.1016/j.vetimm.2012.11.008. Epub 2012 Nov 20. Vet Immunol Immunopathol. 2013. PMID: 23219158 Free PMC article. Review. - SELENOPROTEINS. CRL2 aids elimination of truncated selenoproteins produced by failed UGA/Sec decoding.
Lin HC, Ho SC, Chen YY, Khoo KH, Hsu PH, Yen HC. Lin HC, et al. Science. 2015 Jul 3;349(6243):91-5. doi: 10.1126/science.aab0515. Science. 2015. PMID: 26138980 Free PMC article. - An affinity-directed protein missile system for targeted proteolysis.
Fulcher LJ, Macartney T, Bozatzi P, Hornberger A, Rojas-Fernandez A, Sapkota GP. Fulcher LJ, et al. Open Biol. 2016 Oct;6(10):160255. doi: 10.1098/rsob.160255. Open Biol. 2016. PMID: 27784791 Free PMC article. - ASB7 regulates spindle dynamics and genome integrity by targeting DDA3 for proteasomal degradation.
Uematsu K, Okumura F, Tonogai S, Joo-Okumura A, Alemayehu DH, Nishikimi A, Fukui Y, Nakatsukasa K, Kamura T. Uematsu K, et al. J Cell Biol. 2016 Oct 10;215(1):95-106. doi: 10.1083/jcb.201603062. Epub 2016 Oct 3. J Cell Biol. 2016. PMID: 27697924 Free PMC article.
References
- Aebersold R. and Mann, M. 2003. Mass spectrometry-based proteomics. Nature 422: 198-207. - PubMed
- Aravind L. and Koonin, E.V. 2000. The U box is a modified RING finger—A common domain in ubiquitination. Curr. Biol. 10: R132-R134. - PubMed
- Aso T., Lane, W.S., Conaway, J.W., and Conaway, R.C. 1995. Elongin (SIII): A multisubunit regulator of elongation by RNA polymerase II. Science 269: 1439-1443. - PubMed
- Bai C., Sen, P., Hofmann, K., Ma, L., Goebl, M., Harper, J.W., and Elledge, S.J. 1996. SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell 86: 263-274. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases