Targeted deletion of mek5 causes early embryonic death and defects in the extracellular signal-regulated kinase 5/myocyte enhancer factor 2 cell survival pathway - PubMed (original) (raw)
doi: 10.1128/MCB.25.1.336-345.2005.
Anita J Merritt, Jan Seyfried, Chun Guo, Emmanouil S Papadakis, Katherine G Finegan, Midori Kayahara, Jill Dixon, Raymond P Boot-Handford, Elizabeth J Cartwright, Ulrike Mayer, Cathy Tournier
Affiliations
- PMID: 15601854
- PMCID: PMC538774
- DOI: 10.1128/MCB.25.1.336-345.2005
Targeted deletion of mek5 causes early embryonic death and defects in the extracellular signal-regulated kinase 5/myocyte enhancer factor 2 cell survival pathway
Xin Wang et al. Mol Cell Biol. 2005 Jan.
Abstract
To elucidate the physiological significance of MEK5 in vivo, we have examined the effect of mek5 gene elimination in mice. Heterozygous mice appear to be healthy and were fertile. However, mek5(-/-) embryos die at approximately embryonic day 10.5 (E10.5). The phenotype of the mek5(-/-) embryos includes abnormal cardiac development as well as a marked decrease in proliferation and an increase in apoptosis in the heart, head, and dorsal regions of the mutant embryos. The absence of MEK5 does not affect cell cycle progression but sensitizes mouse embryonic fibroblasts (MEFs) to the ability of sorbitol to enhance caspase 3 activity. Further studies with mek5(-/-) MEFs indicate that MEK5 is required for mediating extracellular signal-regulated kinase 5 (ERK5) activation and for the regulation of the transcriptional activity of myocyte enhancer factor 2. Overall, this is the first study to rigorously establish the role of MEK5 in vivo as an activator of ERK5 and as an essential regulator of cell survival that is required for normal embryonic development.
Figures
FIG. 1.
Strategy for the targeted disruption of the mek5 gene. (A) The genomic region at the mek5 locus, the mek5 targeting vector, and the predicted structure of the mutated mek5 gene are depicted. Restriction enzyme sites are indicated (B, BamH1; RI, EcoRI; H, HindIII). White boxes are mek5 exons. The black box is the β-Gal neomycin cassette (LZ-Neo). (B) Southern blotting analysis of HindIII-restricted genomic DNA prepared from ES cell clones indicates the presence of wild-type (+/+) and heterozygous (+/−) genotypes. The blot was probed with a random-primed 32P-labeled mouse MEK5 genomic probe (see hatched box in panel A). (C) Genomic DNAs isolated from mouse tails were amplified by two separate PCRs with primers specific for the mek5 gene (P1 + P2) and for the LZ-Neo cassette (P3 + P4).
FIG. 2.
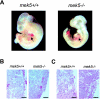
Analysis of MEK5-deficient embryos. (A) A lateral view of freshly isolated wild-type (mek5+/+) and homozygous (_mek5_−/−) E10.5 embryos taken under a light microscope demonstrates retarded development of the head, limbs, and heart in mutant embryos. Arrows point to the heart regions. (B and C) Hematoxylin and eosin staining of sagittal sections of E9.5 (B) and E10.5 (C) mek5+/+ and _mek5_−/− placentas. Scale bar, 50 μm.
FIG. 3.
Expression of MEK5 in embryos and adult tissues. (A) Whole-mount immunohistochemistry to β-Gal in an E9.5 _mek5_−/− embryo demonstrates the specific expression of MEK5 at this early stage of development. Enlarged views of the heart (i) and the tips of the tails (ii) are shown. Scale bars, 50 μm. (B) MEK5 expression is highest in brain, heart, and skeletal muscle tissue. Homozygous (+/+) and heterozygous (+/−) adult mouse tissue extracts (50 μg) were analyzed for β-Gal expression by immunoblot analysis with a specific polyclonal antibody to β-Gal. Protein loading was monitored by India ink staining.
FIG. 4.
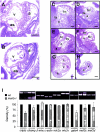
_mek5_−/− embryos display abnormal development of the heart. Hematoxylin and eosin staining of sagittal sections of E9.5 mek5+/+ (A) and _mek5_−/− (B) and E10.5 mek5+/+ (C, E, and G) and _mek5_−/− (D, F, and H) embryos. The spiral septum of the outflow tract displays little or no septal development in _mek5_−/− embryos (D) compared to wild-type embryos (C). The mutant heart displays little evidence of endocardial cushion tissue formation in the atrioventricular canal (compare panels E and F). The trabeculation of both the bulbus cordis and the common ventricular chamber is highly disorganized in _mek5_−/− embryos (D and H) compared to wild-type embryos (C and G). Abbreviations: a, common atrial chamber; as, aortic sac; bc, bulbus cordis; bv, bulbo-ventricular canal; c, cushion tissue; t, trabeculae; ta, truncus arteriosus; v, common ventricular chamber. Scale bars, 10 μm. (I) Semiquantitative RT-PCR analysis of cardiac-related transcripts expressed in wild type (+/+) and mutant (−/−) heart embryos. PCR amplifications were performed with cDNAs synthesized from RNA isolated from E9.5 embryonic hearts. PCR products were quantitated under UV illumination. The results normalized to the levels of amplification of β-actin are expressed as the percentage of expression of the gene in wild-type heart embryos. The data correspond to the means ± standard errors of two independent experiments. Abbreviations are as follows: β-mhc, β-myosin heavy chain; _c_-actin, cardiac α-actin; mlc2, myosin light chain 2.
FIG. 5.
_mek5_−/− embryos exhibit defects in cell proliferation and cell death. Sections of the heart, head, and dorsal regions stained with BrdU (A) reveal decreased cell proliferation in homozygous (_mek5_−/−) compared to wild-type (mek5+/+) E10.5 embryos. TUNEL staining indicates increased apoptosis in the heart, head, and dorsal regions of the _mek5_−/− E9.5 (B) and E10.5 (C) embryos. Scale bars, 25 μm.
FIG. 6.
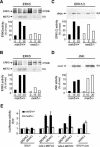
MEK5 is implicated in cell survival. (A) MEF extracts (50 μg) were analyzed for ΜΕΚ5 and ERK5 expression by immunoblot analysis with specific polyclonal anti-MEK5 and anti-ERK5 antibodies. The detection of tubulin expression was performed to monitor protein loading. Asterisks indicate nonspecific bands. (B) Seventy percent confluent MEFs were serum starved for 48 h prior to being stimulated with 10% fetal bovine serum for 24 h. Cell proliferation was assessed by fluorescence-activated cell sorter analysis to follow BrdU incorporation. The percentage of fibroblasts present in the S phase of the cell cycle is indicated. The figure shows data representative of the results from three independent experiments. − serum, without serum; + serum, with serum. (C) Wild-type and _mek5_−/− fibroblasts were incubated for 6 h with 500 mM sorbitol or exposed to UV radiation (60 J/m2) followed by incubation for 16 h. Caspase 3 activity was monitored by using the carboxyfluorescein FLICA apoptosis detection kit. The data, expressed as units of fluorescence, correspond to the means ± standard errors of the results from two independent experiments.
FIG. 7.
Disruption of the mek5 gene prevents ERK5 activation and the transcriptional regulation of MEF2 factors. Wild type (+/+) and homozygous knockout (−/−) mek5 MEFs were treated with EGF (50 ng/ml) (A, C) or sorbitol (300 mM) (B, D) for the times indicated (in minutes). Endogenous ERK5 (A, B), ERK1/2 (C), and JNK (D) activity was measured by protein kinase assay (KA) in the presence of [γ-32P]ATP. The radioactivity incorporated into GST-MEF2C, GST-cMyc, or GST-cJun was quantitated after SDS-PAGE by PhosphorImager analysis. The presence of ERK5 in the immune complexes was detected by immunoblot analysis (IP/WB). Data representative of the results from three independent experiments are shown. (E) Fibroblasts were transiently transfected as described in Materials and Methods. MEF2 and cJun transcriptional activity was measured by the dual-luciferase reporter assay system. Firefly luciferase activity was normalized to that of Renilla luciferase and expressed as change relative to the control (−). The data correspond to the means ± standard errors of three independent experiments performed in duplicate.
Similar articles
- A novel role for extracellular signal-regulated kinase 5 and myocyte enhancer factor 2 in medulloblastoma cell death.
Sturla LM, Cowan CW, Guenther L, Castellino RC, Kim JY, Pomeroy SL. Sturla LM, et al. Cancer Res. 2005 Jul 1;65(13):5683-9. doi: 10.1158/0008-5472.CAN-04-2283. Cancer Res. 2005. PMID: 15994942 - A novel mitogen-activated protein kinase docking site in the N terminus of MEK5alpha organizes the components of the extracellular signal-regulated kinase 5 signaling pathway.
Seyfried J, Wang X, Kharebava G, Tournier C. Seyfried J, et al. Mol Cell Biol. 2005 Nov;25(22):9820-8. doi: 10.1128/MCB.25.22.9820-9828.2005. Mol Cell Biol. 2005. PMID: 16260599 Free PMC article. - Transcriptional regulation of tissue-specific genes by the ERK5 mitogen-activated protein kinase.
Sohn SJ, Li D, Lee LK, Winoto A. Sohn SJ, et al. Mol Cell Biol. 2005 Oct;25(19):8553-66. doi: 10.1128/MCB.25.19.8553-8566.2005. Mol Cell Biol. 2005. PMID: 16166637 Free PMC article. - Oncogenic signaling of MEK5-ERK5.
Hoang VT, Yan TJ, Cavanaugh JE, Flaherty PT, Beckman BS, Burow ME. Hoang VT, et al. Cancer Lett. 2017 Apr 28;392:51-59. doi: 10.1016/j.canlet.2017.01.034. Epub 2017 Jan 30. Cancer Lett. 2017. PMID: 28153789 Free PMC article. Review. - Pathophysiological Impact of the MEK5/ERK5 Pathway in Oxidative Stress.
Tusa I, Menconi A, Tubita A, Rovida E. Tusa I, et al. Cells. 2023 Apr 13;12(8):1154. doi: 10.3390/cells12081154. Cells. 2023. PMID: 37190064 Free PMC article. Review.
Cited by
- Analysis of Nkx3.1:Cre-driven Erk5 deletion reveals a profound spinal deformity which is linked to increased osteoclast activity.
Loveridge CJ, van 't Hof RJ, Charlesworth G, King A, Tan EH, Rose L, Daroszewska A, Prior A, Ahmad I, Welsh M, Mui EJ, Ford C, Salji M, Sansom O, Blyth K, Leung HY. Loveridge CJ, et al. Sci Rep. 2017 Oct 16;7(1):13241. doi: 10.1038/s41598-017-13346-8. Sci Rep. 2017. PMID: 29038439 Free PMC article. - Statin regulated ERK5 stimulates tight junction formation and reduces permeability in human cardiac endothelial cells.
Wilkinson EL, Sidaway JE, Cross MJ. Wilkinson EL, et al. J Cell Physiol. 2018 Jan;233(1):186-200. doi: 10.1002/jcp.26064. Epub 2017 Aug 3. J Cell Physiol. 2018. PMID: 28639275 Free PMC article. - Targeted deletion of the ERK5 MAP kinase impairs neuronal differentiation, migration, and survival during adult neurogenesis in the olfactory bulb.
Li T, Pan YW, Wang W, Abel G, Zou J, Xu L, Storm DR, Xia Z. Li T, et al. PLoS One. 2013 Apr 22;8(4):e61948. doi: 10.1371/journal.pone.0061948. Print 2013. PLoS One. 2013. PMID: 23630619 Free PMC article. - Aberrant MEK5/ERK5 signalling contributes to human colon cancer progression via NF-κB activation.
Simões AE, Pereira DM, Gomes SE, Brito H, Carvalho T, French A, Castro RE, Steer CJ, Thibodeau SN, Rodrigues CM, Borralho PM. Simões AE, et al. Cell Death Dis. 2015 Apr 9;6(4):e1718. doi: 10.1038/cddis.2015.83. Cell Death Dis. 2015. PMID: 25855966 Free PMC article. - Putative Animal Models of Restless Legs Syndrome: A Systematic Review and Evaluation of Their Face and Construct Validity.
Silvani A, Ghorayeb I, Manconi M, Li Y, Clemens S. Silvani A, et al. Neurotherapeutics. 2023 Jan;20(1):154-178. doi: 10.1007/s13311-022-01334-4. Epub 2022 Dec 19. Neurotherapeutics. 2023. PMID: 36536233 Free PMC article.
References
- Abe, J., M. Kusuhara, R. J. Ulevitch, B. C. Berk, and J.-D. Lee. 1996. Big mitogen-activated protein kinase 1 (BMK1) is a redox-sensitive kinase. J. Biol. Chem. 271:16586-16590. - PubMed
- Chao, T.-H., M. Hayashi, R. I. Tapping, Y. Kato, and J.-D. Lee. 1999. MEKK3 directly regulates MEK5 activity as part of the big mitogen-activated protein kinase 1 (BMK1) signaling pathway. J. Biol. Chem. 274:36035-36038. - PubMed
- Deacon, K., and J. L. Blank. 1999. MEK kinase 3 directly activates MKK6 and MKK7, specific activators of the p38 and c-Jun NH2-terminal kinases. J. Biol. Chem. 274:16604-16610. - PubMed
- English, J. M., C. A. Vanderbilt, S. Xu, S. Marcus, and M. H. Cobb. 1995. Isolation of MEK5 and differential expression of alternatively spliced forms. J. Biol. Chem. 270:28897-28902. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous