TBX21: a functional variant predicts improvement in asthma with the use of inhaled corticosteroids - PubMed (original) (raw)
Clinical Trial
. 2004 Dec 28;101(52):18099-104.
doi: 10.1073/pnas.0408532102. Epub 2004 Dec 16.
Affiliations
- PMID: 15604153
- PMCID: PMC539815
- DOI: 10.1073/pnas.0408532102
Clinical Trial
TBX21: a functional variant predicts improvement in asthma with the use of inhaled corticosteroids
Kelan G Tantisira et al. Proc Natl Acad Sci U S A. 2004.
Abstract
TBX21 encodes for the transcription factor T-bet (T-box expressed in T cells), which influences naive T lymphocyte development and has been implicated in asthma pathogenesis. Specifically, the T-bet knockout mouse spontaneously develops airway hyperresponsiveness and other changes consistent with asthma. Because airway responsiveness is moderated by the use of inhaled corticosteroids in asthma, it is conceivable that genetic variation in TBX21 may alter asthma phenotypes in a treatment-specific fashion. Here we demonstrate that the nonsynonymous variation in TBX21 coding for replacement of histidine 33 with glutamine is associated with significant improvement in the PC(20) (a measure of airway responsiveness) of asthmatic children in a large clinical trial spanning 4 years. We note that this increase occurs only in the children randomized to inhaled corticosteroids and that it dramatically enhances the overall improvement in PC(20) associated with inhaled corticosteroid usage. The average PC(20) at trial end for subjects on inhaled corticosteroids possessing a variant allele was in the normal range for nonasthmatics. In cellular models, we show that the TBX21 variant increases T helper 1 and decreases T helper 2 cytokine expression comparably with wild type. TBX21 may thus be an important determinant pharmacogenetic response to the therapy of asthma with inhaled corticosteroids.
Figures
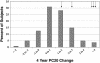
Fig. 1.
Distribution of PC20 response over time. The 4-year change in airway responsiveness in CAMP, as measured by log-transformed PC20, to inhaled corticosteroid therapy varies by individual and is approximately normally distributed. This finding suggests that factors other than treatment, including genetics, may have contributed to the treatment response. Arrows indicate the individuals containing the H33Q variant allele.
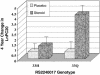
Fig. 2.
The 4-year change in log-transformed PC20 stratified by treatment group assignment and H33Q genotype. Steroid usage by itself is associated with an improvement in the PC20 over time (P < 0.001), whereas the rs2240017 genotype is not (P = 0.90). However, individuals on corticosteroids who also possessed a minor allele encoding for glutamine (33Q) demonstrated dramatic improvement in their PC20 over and above that associated with corticosteroids alone (interaction, P = 0.0002). Values shown are mean ± SE.
Fig. 3.
Geometric mean value of PC20 at various time points over the 4-year follow-up, stratified by treatment group assignment and H33Q genotype. The mean PC20 of individuals on corticosteroids who also possessed a copy of the 33Q variant improved significantly by the end of the first year of the study and continued to improve substantially with time and corticosteroid usage. The mean PC20 value at 4 years is within the range normally ascribed to individuals without the diagnosis of asthma.
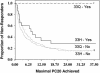
Fig. 4.
Maximal PC20 dose achieved, stratified by treatment group assignment and H33Q genotype. Despite similar PC20 values at baseline, each of the individuals on corticosteroids who also possessed a copy of the 33Q variant fully normalized his PC20, exceeding the upper limits of methacholine testing at least once during the 4-year follow-up. In the other groups, few individuals ever normalized PC20.
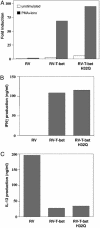
Fig. 5.
Functional characteristics of H32Q, the mouse analog of H33Q. (A) The H32Q polymorphism is equally effective in the transactivation of the IFN-γ gene regulatory region. T-bet or the H32Q version of T-bet were cotransfected into IL-4 cells with an IFN-γ promoter reporter gene and a β-gal reporter gene. Relative luciferase activity was assayed, standardized with β-gal activity, and shown as fold induction. (B and C) H32Q was as effective at modifying cytokine gene expression in primary T cells as wt T-bet. CD4+ T cells were transduced with GFP-RV, RV-T-bet, and RV-T-bet H32Q. Cells were expanded for 5 days, sorted for GFP, and restimulated with anti-CD3 for 24 hours. ELISA was performed to measure IFN-γ and IL-13 production. The results are from three independent experiments. TB, wt T-bet.
Fig. 6.
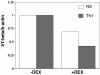
Dexamethasone represses the expression of T-bet in human Th cells. Human CD4 T cells isolated from peripheral blood by using microbeads (Miltenyi Biotec) were stimulated with plate-bound anti-CD3 and anti-CD28 under nonpolarizing (NS) and Th1-skewed conditions in the absence or presence of 10–7 M dexamethasone (DEX). Relative gene transcripts for human T-bet were measured by real time RT-PCR. In both groups, glucocorticoids inhibited the induction T-bet.
Similar articles
- Pharmacogenetic study of the effects of NK2R G231E G>A and TBX21 H33Q C>G polymorphisms on asthma control with inhaled corticosteroid treatment.
Ye YM, Lee HY, Kim SH, Jee YK, Lee SK, Lee SH, Park HS. Ye YM, et al. J Clin Pharm Ther. 2009 Dec;34(6):693-701. doi: 10.1111/j.1365-2710.2009.01054.x. J Clin Pharm Ther. 2009. PMID: 20175803 - Genetic variation of TBX21 gene increases risk of asthma and its severity in Indian children.
Sharma N, Jaiswal I, Mandal RK, Phadke SR, Awasthi S. Sharma N, et al. J Hum Genet. 2014 Aug;59(8):437-43. doi: 10.1038/jhg.2014.52. Epub 2014 Jul 24. J Hum Genet. 2014. PMID: 25056814 - Genome-wide association identifies the T gene as a novel asthma pharmacogenetic locus.
Tantisira KG, Damask A, Szefler SJ, Schuemann B, Markezich A, Su J, Klanderman B, Sylvia J, Wu R, Martinez F, Boushey HA, Chinchilli VM, Mauger D, Weiss ST, Israel E; SHARP Investigators. Tantisira KG, et al. Am J Respir Crit Care Med. 2012 Jun 15;185(12):1286-91. doi: 10.1164/rccm.201111-2061OC. Epub 2012 Apr 26. Am J Respir Crit Care Med. 2012. PMID: 22538805 Free PMC article. Clinical Trial. - Combination of inhaled long-acting beta2-agonists and inhaled steroids versus higher dose of inhaled steroids in children and adults with persistent asthma.
Greenstone IR, Ni Chroinin MN, Masse V, Danish A, Magdalinos H, Zhang X, Ducharme FM. Greenstone IR, et al. Cochrane Database Syst Rev. 2005 Oct 19;(4):CD005533. doi: 10.1002/14651858.CD005533. Cochrane Database Syst Rev. 2005. PMID: 16235409 Updated. Review. - Effects of genetic factors to inhaled corticosteroid response in children with asthma: a literature review.
Duong-Thi-Ly H, Nguyen-Thi-Thu H, Nguyen-Hoang L, Nguyen-Thi-Bich H, Craig TJ, Duong-Quy S. Duong-Thi-Ly H, et al. J Int Med Res. 2017 Dec;45(6):1818-1830. doi: 10.1177/0300060516683877. Epub 2017 Jan 25. J Int Med Res. 2017. PMID: 29251255 Free PMC article. Review.
Cited by
- Relationship between the benefits of suplatast tosilate, a Th2 cytokine inhibitor, and gene polymorphisms in children with bronchial asthma.
Matsui E, Shinoda S, Fukutomi O, Kaneko H, Fukao T, Kondo N. Matsui E, et al. Exp Ther Med. 2010 Nov;1(6):977-982. doi: 10.3892/etm.2010.149. Epub 2010 Sep 15. Exp Ther Med. 2010. PMID: 22993628 Free PMC article. - Genetic associations of the response to inhaled corticosteroids in asthma: a systematic review.
Keskin O, Farzan N, Birben E, Akel H, Karaaslan C, Maitland-van der Zee AH, Wechsler ME, Vijverberg SJ, Kalayci O. Keskin O, et al. Clin Transl Allergy. 2019 Jan 9;9:2. doi: 10.1186/s13601-018-0239-2. eCollection 2019. Clin Transl Allergy. 2019. PMID: 30647901 Free PMC article. Review. - Multiomics analysis identifies BIRC3 as a novel glucocorticoid response-associated gene.
Kan M, Diwadkar AR, Shuai H, Joo J, Wang AL, Ong MS, Sordillo JE, Iribarren C, Lu MX, Hernandez-Pacheco N, Perez-Garcia J, Gorenjak M, Potočnik U, Burchard EG, Pino-Yanes M, Wu AC, Himes BE. Kan M, et al. J Allergy Clin Immunol. 2022 Jun;149(6):1981-1991. doi: 10.1016/j.jaci.2021.11.025. Epub 2021 Dec 28. J Allergy Clin Immunol. 2022. PMID: 34971648 Free PMC article. - Pharmacogenetics and interstitial lung disease.
Oldham JM, Noth I, Martinez FJ. Oldham JM, et al. Curr Opin Pulm Med. 2016 Sep;22(5):456-65. doi: 10.1097/MCP.0000000000000289. Curr Opin Pulm Med. 2016. PMID: 27253772 Free PMC article. Review. - Pharmacogenomic Response of Inhaled Corticosteroids for the Treatment of Asthma: Considerations for Therapy.
Cazzola M, Rogliani P, Calzetta L, Matera MG. Cazzola M, et al. Pharmgenomics Pers Med. 2020 Aug 4;13:261-271. doi: 10.2147/PGPM.S231471. eCollection 2020. Pharmgenomics Pers Med. 2020. PMID: 32801837 Free PMC article. Review.
References
- Barnes, P. J. (1996) Am. J. Respir. Crit. Care Med. 154, S21–S27. - PubMed
- Bousquet, J. (2000) Clin. Exp. Allergy 30, Suppl. 1, 2–5. - PubMed
- Lemanske, R. F., Jr., & Allen, D. B. (1997) Am. J. Respir. Crit. Care Med. 156, 685–687. - PubMed
- Malmstrom, K., Rodriguez-Gomez, G., Guerra, J., Villaran, C., Pineiro, A., Wei, L. X., Seidenberg, B. C. & Reiss, T. F. (1999) Ann. Intern. Med. 130, 487–495. - PubMed
- Szefler, S. J., Martin, R. J., King, T. S., Boushey, H. A., Cherniack, R. M., Chinchilli, V. M., Craig, T. J., Dolovich, M., Drazen, J. M., Fagan, J. K., et al. (2002) J. Allergy Clin. Immunol. 109, 410–418. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- N01HR16048/HR/NHLBI NIH HHS/United States
- HR16046/HR/NHLBI NIH HHS/United States
- N01 HR16044/HR/NHLBI NIH HHS/United States
- U19 AI031541/AI/NIAID NIH HHS/United States
- HR16051/HR/NHLBI NIH HHS/United States
- HR16052/HR/NHLBI NIH HHS/United States
- HR16048/HR/NHLBI NIH HHS/United States
- N01HR16050/HR/NHLBI NIH HHS/United States
- HR16049/HR/NHLBI NIH HHS/United States
- HR16045/HR/NHLBI NIH HHS/United States
- P01 AI031541/AI/NIAID NIH HHS/United States
- N01HR16051/HR/NHLBI NIH HHS/United States
- N01HR16044/HR/NHLBI NIH HHS/United States
- AI31541/AI/NIAID NIH HHS/United States
- N01HR16052/HR/NHLBI NIH HHS/United States
- N01-HR-16049/HR/NHLBI NIH HHS/United States
- U01 HL65899/HL/NHLBI NIH HHS/United States
- N01HR16045/HR/NHLBI NIH HHS/United States
- U01 HL065899/HL/NHLBI NIH HHS/United States
- N01HR16047/HR/NHLBI NIH HHS/United States
- HR16050/HR/NHLBI NIH HHS/United States
- N01HR16046/HR/NHLBI NIH HHS/United States
- N01HR16049/HR/NHLBI NIH HHS/United States
- HR16047/HR/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases