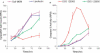Sequence-dependent cytotoxicity of second-generation oligonucleotides - PubMed (original) (raw)
. 2004 Dec 16;32(22):6585-94.
doi: 10.1093/nar/gkh997. Print 2004.
Affiliations
- PMID: 15604456
- PMCID: PMC545465
- DOI: 10.1093/nar/gkh997
Sequence-dependent cytotoxicity of second-generation oligonucleotides
Denis Drygin et al. Nucleic Acids Res. 2004.
Abstract
In this study, we have examined the potential of second-generation antisense chimeric 2'-O-(2-methoxy)ethyl/DNA phosphorothioate oligonucleotides (ONs) to affect cell growth through non-antisense mechanisms. Evaluation of a series of ONs demonstrated that only a small number were cytotoxic at concentrations close to those required for antisense activity. Toxicity of the ONs appeared to be sequence dependent and could be affected by base and backbone modifications. Caspase-3 activation occurs with some ONs and it is most likely secondary to necrosis rather than apoptosis, since cells treated with toxic ONs did not show chromatin condensation, but did exhibit high-extracellular lactate dehydrogenase activity. Caspase-3 activation does not correlate with and appears not to be required for the inhibition of cell proliferation. Toxicity was only observed when ONs were delivered intracellularly. The mechanism by which one of the most cytotoxic ON produces cytotoxicity was investigated in more detail. Treatment with the cytotoxic ON caused disruption of lysosomes and Pepstatin A, a specific inhibitor of aspartic proteases, reduced the cytotoxicity of the ON. Reduction of lysosomal aspartic protease cathepsin D by prior treatment with cathepsin D-specific antisense ON did not attenuate the cytotoxicity, suggesting that other aspartic proteases play a crucial role in the cellular proliferation inhibition by ONs.
Figures
Figure 1
Dose–response of gene downregulation versus cytotoxicity of ONs in A549 cells. Cells were transfected with various amounts of either ISIS 126965 or ISIS 129696 for toxicity studies or ISIS 116847 for PTEN downregulation analysis (Lipofectin-mediated transfection or electroporation). Forty-eight hours after transfection, the cell number was measured with CyQUANT cell proliferation kit and PTEN mRNA level was determined using Taqman quantitative RT–PCR. Values for UTC are designated as 100%.
Figure 2
Time course of cell growth and caspase-3 activation after transfection with ONs. A549 cells were treated with Opti-Mem alone, Lipofectin (9 μg/ml) in Opti-MEM or with 0.3 μM of ISIS 126965 or ISIS 129696 in the presence of Lipofectin (3 μg/ml per 100 nM ON). Cells were collected at 12, 24, 36, 48, 74 and 98 h after the beginning of the transfection. (A) Cell number was measured with CyQUANT cell proliferation kit. (B) Caspase-3 activity was measured using HTS caspase-3 activity assay. The data are presented in relative fluorescence units (RFU).
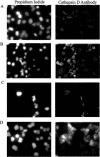
Figure 3
Treatment of A549 cells with staurosporine or ISIS 126965 releases cathepsin D from lysosomes. A549 cells (A) untreated or treated with (B) 250 nM of staurosporine, (C) 0.3 μM of ISIS 130358 in the presence of Lipofectin (3 μg/ml per 100 nM ON) or (D) 0.3 μM of ISIS 126965 in the presence of Lipofectin (3 μg/ml per 100 nM ON), were simultaneously stained 24 h after transfection with propidium iodide and anti-cathepsin D FITC-labeled antibody and examined by fluorescent microscopy.
Similar articles
- Aryl hydrocarbon receptor modulation of tumor necrosis factor-alpha-induced apoptosis and lysosomal disruption in a hepatoma model that is caspase-8-independent.
Caruso JA, Mathieu PA, Joiakim A, Zhang H, Reiners JJ Jr. Caruso JA, et al. J Biol Chem. 2006 Apr 21;281(16):10954-67. doi: 10.1074/jbc.M508383200. Epub 2006 Jan 30. J Biol Chem. 2006. PMID: 16446372 - Regulation of a novel pathway for cell death by lysosomal aspartic and cysteine proteinases.
Isahara K, Ohsawa Y, Kanamori S, Shibata M, Waguri S, Sato N, Gotow T, Watanabe T, Momoi T, Urase K, Kominami E, Uchiyama Y. Isahara K, et al. Neuroscience. 1999;91(1):233-49. doi: 10.1016/s0306-4522(98)00566-1. Neuroscience. 1999. PMID: 10336074 - Site and mechanism of antisense inhibition by C-5 propyne oligonucleotides.
Moulds C, Lewis JG, Froehler BC, Grant D, Huang T, Milligan JF, Matteucci MD, Wagner RW. Moulds C, et al. Biochemistry. 1995 Apr 18;34(15):5044-53. doi: 10.1021/bi00015a015. Biochemistry. 1995. PMID: 7536034 - In vitro toxicology and pharmacokinetics of antisense oligonucleotides.
Crooke RM. Crooke RM. Anticancer Drug Des. 1991 Dec;6(6):609-46. Anticancer Drug Des. 1991. PMID: 1772572 Review. - Pathophysiological Roles of Intracellular Proteases in Neuronal Development and Neurological Diseases.
Yagami T, Yamamoto Y, Koma H. Yagami T, et al. Mol Neurobiol. 2019 May;56(5):3090-3112. doi: 10.1007/s12035-018-1277-4. Epub 2018 Aug 10. Mol Neurobiol. 2019. PMID: 30097848 Review.
Cited by
- Stable transmission of targeted gene modification using single-stranded oligonucleotides with flanking LNAs.
Andrieu-Soler C, Casas M, Faussat AM, Gandolphe C, Doat M, Tempé D, Giovannangeli C, Behar-Cohen F, Concordet JP. Andrieu-Soler C, et al. Nucleic Acids Res. 2005 Jul 7;33(12):3733-42. doi: 10.1093/nar/gki686. Print 2005. Nucleic Acids Res. 2005. PMID: 16002788 Free PMC article. - Acute Neurotoxicity of Antisense Oligonucleotides After Intracerebroventricular Injection Into Mouse Brain Can Be Predicted from Sequence Features.
Hagedorn PH, Brown JM, Easton A, Pierdomenico M, Jones K, Olson RE, Mercer SE, Li D, Loy J, Høg AM, Jensen ML, Gill M, Cacace AM. Hagedorn PH, et al. Nucleic Acid Ther. 2022 Jun;32(3):151-162. doi: 10.1089/nat.2021.0071. Epub 2022 Feb 14. Nucleic Acid Ther. 2022. PMID: 35166597 Free PMC article. - Regulation of human microglial gene expression and function via RNAase-H active antisense oligonucleotides in vivo in Alzheimer's disease.
Vandermeulen L, Geric I, Fumagalli L, Kreir M, Lu A, Nonneman A, Premereur J, Wolfs L, Policarpo R, Fattorelli N, De Bondt A, Van Den Wyngaert I, Asselbergh B, Fiers M, De Strooper B, d'Ydewalle C, Mancuso R. Vandermeulen L, et al. Mol Neurodegener. 2024 Apr 24;19(1):37. doi: 10.1186/s13024-024-00725-9. Mol Neurodegener. 2024. PMID: 38654375 Free PMC article. - Establishment of a Predictive In Vitro Assay for Assessment of the Hepatotoxic Potential of Oligonucleotide Drugs.
Sewing S, Boess F, Moisan A, Bertinetti-Lapatki C, Minz T, Hedtjaern M, Tessier Y, Schuler F, Singer T, Roth AB. Sewing S, et al. PLoS One. 2016 Jul 21;11(7):e0159431. doi: 10.1371/journal.pone.0159431. eCollection 2016. PLoS One. 2016. PMID: 27442522 Free PMC article. - Intracerebral Infusion of Antisense Oligonucleotides Into Prion-infected Mice.
Nazor Friberg K, Hung G, Wancewicz E, Giles K, Black C, Freier S, Bennett F, Dearmond SJ, Freyman Y, Lessard P, Ghaemmaghami S, Prusiner SB. Nazor Friberg K, et al. Mol Ther Nucleic Acids. 2012 Feb 7;1(2):e9. doi: 10.1038/mtna.2011.6. Mol Ther Nucleic Acids. 2012. PMID: 23344724 Free PMC article.
References
- Levin A.A., Henry,S.P., Monteith,D. and Templin,M.V. (2001) Toxicity of antisense oligonucleotides. In Crooke,S.T. (ed.), Antisense Drug Technology: Principles, Strategies and Applications. Marcel Dekker, Inc., New York, NY, pp. 201–269.
- Dorr F.A., Glover,J.G. and Kwoh,T.J. (2001) Clinical safety of phosphorothioate oligonucleotides. In Crooke,S.T. (ed.), Antisense Drug Technology: Principles, Strategies, and Applications. Marcel Dekker, Inc., New York, NY, pp. 269–318.
- Balakireva L.A., Levashova,Z.B., Chroboczek,J. and Vlassov,V.V. (1997) Rapid sequence-independent cellular response to oligodeoxynucleotides. FEBS Lett., 400, 267–270. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous