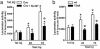Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum - PubMed (original) (raw)
. 2005 Jan 11;102(2):491-6.
doi: 10.1073/pnas.0408305102. Epub 2004 Dec 17.
Vincent Pascoli, Per Svenningsson, Surojit Paul, Hervé Enslen, Jean-Christophe Corvol, Alexandre Stipanovich, Jocelyne Caboche, Paul J Lombroso, Angus C Nairn, Paul Greengard, Denis Hervé, Jean-Antoine Girault
Affiliations
- PMID: 15608059
- PMCID: PMC544317
- DOI: 10.1073/pnas.0408305102
Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum
Emmanuel Valjent et al. Proc Natl Acad Sci U S A. 2005.
Abstract
Many drugs of abuse exert their addictive effects by increasing extracellular dopamine in the nucleus accumbens, where they likely alter the plasticity of corticostriatal glutamatergic transmission. This mechanism implies key molecular alterations in neurons in which both dopamine and glutamate inputs are activated. Extracellular signal-regulated kinase (ERK), an enzyme important for long-term synaptic plasticity, is a good candidate for playing such a role. Here, we show in mouse that d-amphetamine activates ERK in a subset of medium-size spiny neurons of the dorsal striatum and nucleus accumbens, through the combined action of glutamate NMDA and D1-dopamine receptors. Activation of ERK by d-amphetamine or by widely abused drugs, including cocaine, nicotine, morphine, and Delta(9)-tetrahydrocannabinol was absent in mice lacking dopamine- and cAMP-regulated phosphoprotein of M(r) 32,000 (DARPP-32). The effects of d-amphetamine or cocaine on ERK activation in the striatum, but not in the prefrontal cortex, were prevented by point mutation of Thr-34, a DARPP-32 residue specifically involved in protein phosphatase-1 inhibition. Regulation by DARPP-32 occurred both upstream of ERK and at the level of striatal-enriched tyrosine phosphatase (STEP). Blockade of the ERK pathway or mutation of DARPP-32 altered locomotor sensitization induced by a single injection of psychostimulants, demonstrating the functional relevance of this regulation. Thus, activation of ERK, by a multilevel protein phosphatase-controlled mechanism, functions as a detector of coincidence of dopamine and glutamate signals converging on medium-size striatal neurons and is critical for long-lasting effects of drugs of abuse.
Figures
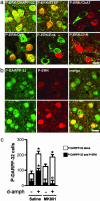
Fig. 1.
_d_-amph-induced ERK activation in MSNs. (a) Double immunolabeling of P-ERK1/2 (red) and the indicated proteins (green) in the shell of the nucleus accumbens, 15 min after i.p. injection of _d_-amph (10 mg/kg) (see also Table 1). ChAT, choline acetyltransferase; Dyn, dynorphin; Enk, enkephalin. (b) Double immunolabeling of P-ERK (red) and phospho-Thr-34-DARPP-32 (P-DARPP-32, green) in vehicle-treated (Upper) or d-ampf-treated (Lower) mice. (c) Fifteen minutes before _d_-amph (+) or saline (-) injection, mice were treated with saline or MK801 (0.1 mg/kg). Neurons immunoreactive for P-DARPP-32 were counted, and the proportion of neurons immunoreactive for both P-DARPP-32 and P-ERK is indicated as filled bars. Virtually all P-ERK-positive neurons were P-DARPP-32-positive (not shown). Data are means ± SEM (neurons per shell field, five mice per group, _d_-amph-treated vs. control: *, P < 0.05; antagonist vs. saline pretreatment: º, P < 0.05). Photographs are single confocal sections. (Scale bars: 10 μm.)
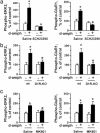
Fig. 2.
Regulation of ERK and GluR1 phosphorylation in the mouse striatum in vivo by _d_-amph. Fifteen minutes after injection of saline (-)or _d_-amph (10 mg/kg) (+), phosphorylation of ERK2 and GluR1 at Ser-845 were quantified in the striatum by immunoblotting in mice pretreated with saline or the D1R antagonist SCH23390 (0.25 mg/kg, i.p.) 30 min before saline or _d_-amph (a), in wild-type (wt) or D1R knockout (D1R-KO) mice (b), or in mice pretreated with saline or the NMDAR antagonist MK801 (0.1 mg/kg) 30 min before saline or _d_-amph (c). Results are expressed as percentages of controls. Data are means ± SEM (five mice per group, _d_-amph-treated vs. control: *, P < 0.05; knockout vs. wild type or antagonist vs. saline pretreatment: º, P < 0.05).
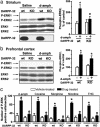
Fig. 3.
Requirement of DARPP-32 in the activation of ERK in the striatum by drugs of abuse. (a) Immunoblot analysis (quantification in Right) of striatal extracts from wild-type (wt) and DARPP-32-knockout (KO) mice 15 min after i.p. injection of saline or _d_-amph (10 mg/kg). (b) Immunoblot analysis of prefrontal cortex extracts as in a. (c) Quantification of P-ERK-positive cells in sections of the nucleus accumbens from wt and DARPP-32-KO mice 10 min after injection of _d_-amph (10 mg/kg, i.p.) or cocaine (20 mg/kg, i.p.), or 20 min after injection of morphine (5 mg/kg, s.c.), nicotine (0.4 mg/kg, s.c.), or THC (1 mg/kg, i.p.), and their respective vehicle-treated controls. Data are means ± SEM (five mice per group, drug-treated vs. control: *, P < 0.05; KO vs. wt: º, P < 0.05).
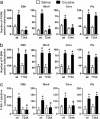
Fig. 4.
DARPP-32 phosphorylation on residue Thr-34 is required for activation of ERK in the striatum by cocaine. (a) The number of P-ERK-positive neurons was quantified in dorsal striatum (DStr), nucleus accumbens (shell and core), and prefrontal cortex (Pfx) in wild-type (wt) and Thr-34 → Ala DARPP-32 (T34A) mutant mice 10 min after i.p. injection of saline or cocaine (20 mg/kg). (b) Same experiment in wild-type and Thr-75 → Ala DARPP-32 (T75A) mutant mice. (c) Phosphorylation of transcription factor Elk-1 Ser-383 was examined in wild-type and Thr-34 → Ala DARPP-32 (T34A) mutant mice 10 min after i.p. injection of saline or cocaine (20 mg/kg). The number of immunofluorescent neuronal nuclei labeled with phosphorylation state-specific antibodies was quantified in dorsal striatum (DStr), nucleus accumbens (shell and core), and prefrontal cortex (Pfx). Data are means ± SEM; six mice per group; treated vs. control: *, P < 0.01; wild type vs. mutant: º, P < 0.05.
Fig. 5.
Role of DARPP-32 and ERK activation in behavioral sensitization. (a) Locomotor activity was measured in response to a first injection (1st inj) of cocaine (20 mg/kg, i.p.) in mice pretreated with vehicle (open bars) or SL327 (30 mg/kg i.p., filled bars), 30 min before cocaine injection. Locomotor activity in response to vehicle injection did not differ between saline-pretreated (145 ± 68¼ turns per 60 min) and SL327-pretreated (119 ± 27¼ turns per 60 min) mice. The response to a test injection of cocaine (Test inj) was measured either 2 days (2d) or 7 days (7d) later. (b) The locomotor effects of the first and test injections of cocaine were examined by using the same protocol in wild-type (wt) and Thr-34 → Ala DARPP-32 (T34A) mutant mice (11 mice per group). *, P < 0.01 test vs. first injection; º, P < 0.01 SL327 vs. saline pretreatment, or mutant vs. wild type.
Fig. 6.
Requirement of DARPP-32 in _d_-amph-induced phosphorylation of STEP and MEK. (a) Regulation of STEP phosphorylation in striatum from wild-type (wt) and DARPP-32-knockout (KO) mice injected with saline or _d_-amph (10 mg/kg, i.p.). Phosphorylation of STEP (P-STEP) is indicated by the upward shift of the 46-kDa isoform (STEP46). STEP46 phosphorylation was expressed as the ratio P-STEP46/total STEP46. (b) Regulation of MEK phosphorylation in striatum from wt and KO mice injected with saline or _d_-amph (10 mg/kg, i.p.). MEK phosphorylation was expressed as percentage of controls. Data are means ± SEM (six mice per group; treated vs. control: *, P < 0.01; treated KO mice vs. wt: º, P < 0.05). (c) Schematic representation of the role of phosphatase regulation in the stimulation of ERK after activation of D1R and NMDAR by acute psychostimulant administration.
Comment in
- DARPP-32: A molecular switch at the nexus of reward pathway plasticity.
Gould TD, Manji HK. Gould TD, et al. Proc Natl Acad Sci U S A. 2005 Jan 11;102(2):253-4. doi: 10.1073/pnas.0408700102. Epub 2005 Jan 4. Proc Natl Acad Sci U S A. 2005. PMID: 15632217 Free PMC article. No abstract available.
Similar articles
- Glutamate regulation of DARPP-32 phosphorylation in neostriatal neurons involves activation of multiple signaling cascades.
Nishi A, Watanabe Y, Higashi H, Tanaka M, Nairn AC, Greengard P. Nishi A, et al. Proc Natl Acad Sci U S A. 2005 Jan 25;102(4):1199-204. doi: 10.1073/pnas.0409138102. Epub 2005 Jan 18. Proc Natl Acad Sci U S A. 2005. PMID: 15657149 Free PMC article. - Dopamine enhancement of NMDA currents in dissociated medium-sized striatal neurons: role of D1 receptors and DARPP-32.
Flores-Hernández J, Cepeda C, Hernández-Echeagaray E, Calvert CR, Jokel ES, Fienberg AA, Greengard P, Levine MS. Flores-Hernández J, et al. J Neurophysiol. 2002 Dec;88(6):3010-20. doi: 10.1152/jn.00361.2002. J Neurophysiol. 2002. PMID: 12466426 - Dopamine D1-dependent trafficking of striatal N-methyl-D-aspartate glutamate receptors requires Fyn protein tyrosine kinase but not DARPP-32.
Dunah AW, Sirianni AC, Fienberg AA, Bastia E, Schwarzschild MA, Standaert DG. Dunah AW, et al. Mol Pharmacol. 2004 Jan;65(1):121-9. doi: 10.1124/mol.65.1.121. Mol Pharmacol. 2004. PMID: 14722243 - Extracellular signal-regulated protein kinases 1 and 2 activation by addictive drugs: a signal toward pathological adaptation.
Pascoli V, Cahill E, Bellivier F, Caboche J, Vanhoutte P. Pascoli V, et al. Biol Psychiatry. 2014 Dec 15;76(12):917-26. doi: 10.1016/j.biopsych.2014.04.005. Epub 2014 Apr 18. Biol Psychiatry. 2014. PMID: 24844603 Review. - Dopaminergic control of synaptic plasticity in the dorsal striatum.
Centonze D, Picconi B, Gubellini P, Bernardi G, Calabresi P. Centonze D, et al. Eur J Neurosci. 2001 Mar;13(6):1071-7. doi: 10.1046/j.0953-816x.2001.01485.x. Eur J Neurosci. 2001. PMID: 11285003 Review.
Cited by
- Rescue of dopamine transporter function in hypoinsulinemic rats by a D2 receptor-ERK-dependent mechanism.
Owens WA, Williams JM, Saunders C, Avison MJ, Galli A, Daws LC. Owens WA, et al. J Neurosci. 2012 Feb 22;32(8):2637-47. doi: 10.1523/JNEUROSCI.3759-11.2012. J Neurosci. 2012. PMID: 22357848 Free PMC article. - STEP61 is a substrate of the E3 ligase parkin and is upregulated in Parkinson's disease.
Kurup PK, Xu J, Videira RA, Ononenyi C, Baltazar G, Lombroso PJ, Nairn AC. Kurup PK, et al. Proc Natl Acad Sci U S A. 2015 Jan 27;112(4):1202-7. doi: 10.1073/pnas.1417423112. Epub 2015 Jan 12. Proc Natl Acad Sci U S A. 2015. PMID: 25583483 Free PMC article. - Genetic evidence implicating DARPP-32 in human frontostriatal structure, function, and cognition.
Meyer-Lindenberg A, Straub RE, Lipska BK, Verchinski BA, Goldberg T, Callicott JH, Egan MF, Huffaker SS, Mattay VS, Kolachana B, Kleinman JE, Weinberger DR. Meyer-Lindenberg A, et al. J Clin Invest. 2007 Mar;117(3):672-82. doi: 10.1172/JCI30413. Epub 2007 Feb 8. J Clin Invest. 2007. PMID: 17290303 Free PMC article. - Dopamine D1 and D3 receptors are differentially involved in cue-elicited cocaine seeking.
Chen L, Xu M. Chen L, et al. J Neurochem. 2010 Jul;114(2):530-41. doi: 10.1111/j.1471-4159.2010.06775.x. Epub 2010 Apr 28. J Neurochem. 2010. PMID: 20456009 Free PMC article. - DARPP-32 mediates the actions of multiple drugs of abuse.
Svenningsson P, Nairn AC, Greengard P. Svenningsson P, et al. AAPS J. 2005 Oct 5;7(2):E353-60. doi: 10.1208/aapsj070235. AAPS J. 2005. PMID: 16353915 Free PMC article. Review.
References
- Di Chiara, G. (1999) Eur. J. Pharmacol. 375, 13-30. - PubMed
- Schultz, W. (2002) Neuron 36, 241-263. - PubMed
- Reynolds, J. N. J. & Wickens, J. R. (2002) Neural Netw. 15, 507-521. - PubMed
- Hyman, S. E. & Malenka, R. C. (2001) Nat. Rev. Neurosci. 2, 695-703. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 MH040899/MH/NIMH NIH HHS/United States
- K02 MH001527/MH/NIMH NIH HHS/United States
- MH40899/MH/NIMH NIH HHS/United States
- R01 MH52711/MH/NIMH NIH HHS/United States
- P01 DA010044/DA/NIDA NIH HHS/United States
- K02 MH01527/MH/NIMH NIH HHS/United States
- DA10044/DA/NIDA NIH HHS/United States
- R01 MH052711/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous