Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway - PubMed (original) (raw)
. 2004 Dec 28;101(52):18117-22.
doi: 10.1073/pnas.0408258102. Epub 2004 Dec 17.
Khadir Raddassi, Kook In Park, Franz-Josef Mueller, Marta Nieto, Yang D Teng, Dan Frenkel, Jianxue Li, Richard L Sidman, Christopher A Walsh, Evan Y Snyder, Samia J Khoury
Affiliations
- PMID: 15608062
- PMCID: PMC536055
- DOI: 10.1073/pnas.0408258102
Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway
Jaime Imitola et al. Proc Natl Acad Sci U S A. 2004.
Abstract
Migration toward pathology is the first critical step in stem cell engagement during regeneration. Neural stem cells (NSCs) migrate through the parenchyma along nonstereotypical routes in a precise directed manner across great distances to injury sites in the CNS, where they might engage niches harboring local transiently expressed reparative signals. The molecular mechanisms for NSC mobilization have not been identified. Because NSCs seem to home similarly to pathologic sites derived from disparate etiologies, we hypothesized that the inflammatory response itself, a characteristic common to all, guides the behavior of potentially reparative cells. As proof of concept, we show that human NSCs migrate in vivo (including from the contralateral hemisphere) toward an infarcted area (a representative CNS injury), where local astrocytes and endothelium up-regulate the inflammatory chemoattractant stromal cell-derived factor 1alpha (SDF-1alpha). NSCs express CXC chemokine receptor 4 (CXCR4), the cognate receptor for SDF-1alpha. Exposure of SDF-1alpha to quiescent NSCs enhances proliferation, promotes chain migration and transmigration, and activates intracellular molecular pathways mediating engagement. CXCR4 blockade abrogates their pathology-directed chain migration, a developmentally relevant mode of tangential migration that, if recapitulated, could explain homing along nonstereotypical paths. Our data implicate SDF-1alpha/CXCR4, representative of the inflammatory milieu characterizing many pathologies, as a pathway that activates NSC molecular programs during injury and suggest that inflammation may be viewed not simply as playing an adverse role but also as providing stimuli that recruit cells with a regenerative homeostasis-promoting capacity. CXCR4 expression within germinal zones suggests that NSC homing after injury and migration during development may invoke similar mechanisms.
Figures
Fig. 1.
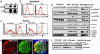
SDF-1α enhances proliferation and induces two forms of migration of hNSCs. (a) Proliferation assay showing significantly increased thymidine incorporation by hNSCs after increasing SDF-1α dosage (cultured in triplicate): 10 ng/ml, P = 0.03; 100 ng/ml, P = 0.01; 1,000 ng/ml, P = 0.0004 compared with control (by Student's t test.) (b Upper) Boyden chamber assay used for assaying migration. hNSCs are allowed to migrate into a fibronectin-coated membrane (green), immersed in medium (orange) containing SDF-1α at different concentrations. The number of cells that cross the membrane and stain with crystal violet (blue) reflects their migratory capacity. (b Lower) Quantification of hNSC migration as measured by optical density (absorbance) of crystal violet after 48 h. There is a significant increase in migration in response to SDF-1α: 500 ng/ml, P < 0.02; 1 μg/ml, P < 0.05, compared with control (by Student's t test). (c) Number of chains migrating from neurospheres. SDF-1α alone significantly increases this number compared with no treatment (No T); P < 0.05 ANOVA. (d) Mean maximal distance migrated per chain/neurosphere: SDF-1α alone had a significant effect compared with untreated (P < 0.05 ANOVA), as did FGF (P < 0.01) and FGF plus SDF-1α (P < 0.001). There was no significant difference between FGF and FGF plus SDF-1α.(e and f) Phase-contrast photomicrograph of neurosphere incubated in FGF (20 ng/ml) alone (e) compared with neurosphere in FGF (20 ng/ml) plus SDF-1α (100 ng/ml) (f), where there appears to be an increase in the number of migrating cells and the complexity, branching, and thickness of migratory chains (arrows in f). (g) Composite dark-field image of neurosphere in bFGF alone compared with one treated with bFGF plus SDF-1α. Such images were used to quantify the Df of chain migration. For bFGF-treated hNSCs, Df = 1.2; for bFGF plus SDF-1α, Df = 1.5 (Df values range from 1 to 2; values approaching 2 denote increasing complexity). The Df for hNSCs exposed to no additives is negligible. (h) Quantification of branching complexity comparing bFGF-exposed with bFGF plus SDF-1α-exposed hNSCs showing a significant increase in branching numbers (P < 0.003). (Branching for hNSCs exposed to no additives is negligible.)
Fig. 2.
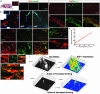
hNSCs express CXCR4, which activates intracellular molecular pathways upon SDF-1α stimulation. (a) Ribonuclease protection assay showing expression of CXCR4 mRNA in two hNSC lines. Lane 1, yeast RNA (control); lane 2, hNSC clone H6 (6, 8, 9); lane 3, hNSC clone CC-2599 (43). L32, housekeeping gene as control for equal loading. (b) FACS analysis of permeabilized cells revealing intracellular CXCR4 in the two above-mentioned hNSC lines. Black dotted histogram represents results from the isotype control antibody; the red histogram represents staining with a monoclonal antibody against human CXCR4. (c) Membrane expression of CXCR4 in three hNSC lines, H6, CC-2599, and HFB2050, compared with purified CD4+ lymphocytes serving as a positive control. (d) Confocal microscopic image of hNSCs dual-immunostained as neurospheres with antibodies to CXCR4 and vimentin. (e) Activation by SDF-1α of hNSC intracellular signaling pathways. hNSCs were exposed to SDF-1α (100 ng/ml) or to FGF plus EGF (20 ng/ml) followed by Western analysis for phosphorylation of p38MAPK, p90Rsk, c-Jun, and extracellular response kinase at the indicated time points. The phosphorylated form (p-) is shown (Upper) for each molecule. (f) The rapid kinetics of paxilin phosphorylation (p-Paxilin) with an increase after 2 and 5 min and a reduction thereafter.
Fig. 3.
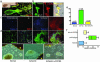
hNSCs migrate to areas of SDF-1α up-regulation after stroke. (a) Mouse brain (coronal view) subjected to unilateral stroke. The infarct, largely a necrotic cavity, is delineated by the blue area. Three areas per slide (boxes 1–3) were analyzed. Boxes 1 and 2 include the penumbra; box 3 is from the contralateral uninjured cortex. The hNSCs depicted here were prelabeled ex vivo with chloromethylbenzamido derivate of 1,1′-dioctadecyl-3,3,3′,3-tetramethylindocarbacyanine perchlorate (DiI) before transplantation. (Inset) Red DiI+ hNSCs have homed to area 1. (b) DiI+ hNSCs (arrows), magnified (Inset), are seen migrating across the corpus callosum (CC) from the contralateral intact hemisphere (site of implantation) to the infarcted hemisphere. (c) DiI fluorescence is specific to hNSCs in ischemic brain (Left) and not seen at the Alexa Fluor 488 wavelength (Center) used for revealing SDF-1α immunoreactivity; merged (Right). (d) Normal contralateral side (box 3) showing absence of DiI+ hNSCs (Left) and only expected meningeal (m) staining of SDF-1α (green) (Center); merge (Right). (e) DiI+ hNSCs that have robustly homed to the penumbra (bottom), some of which have responded to this neurogenic niche by differentiating into neurons (identified by an anti-human-specific NF antibody, an independent marker for hNSC-derived cells) (f and g). SDF-1α immunoreactivity (green) is robust throughout the penumbra. Boxed area in e is viewed at higher power as a merged image in h where a confocal microscopic 3D reconstruction shows the hNSCs (red) intertwined intimately with the abundant SDF-1α-expressing cells (green). The boxed area in h is shown at higher power in i via the red channel capturing a residual classic elongated migratory profile of some DiI+ hNSCs. (j) High-resolution quantitative 2.5-dimensional imaging of hNSCs homing to SDF-1α-enriched areas. Topographical view of multiple areas (n = 30) measured by confocal profile intensity. hNSC values are in gray scale; maximal SDF-1α expression is represented in color. hNSCs colocalize with (home to) areas of high (Upper) but not low (Lower) SDF-1α.(k) Correlation of SDF-1α expression in injured cortex and number of migrating hNSCs (r = 0.679; P < 0.0001). (l upper) Contralateral normal parenchyma showing normal astrocytes (GFAP; red) with no SDF-1α (green) coimmunostaining. (l lower) Infarcted side showing both an increase in GFAP-immunoreactive cells (red) with thick processes (suggestive of reactive astrocytes) and an increase in SDF-1α+ (green) coimmunostaining. Merged images (yellow) suggest that the reactive astrocytes are coexpressing SDF-1α. Colocalization of GFAP (red) and SDF-1α (green) is confirmed by optical dissection and orthogonal reconstruction of the confocal image (o), showing intracellular localization of SDF-1α in two representative reactive astrocytes (arrows).
Fig. 4.
Migration in vitro of NSCs from subventricular zone toward explants from ischemic brain is mediated by SDF-1α–CXCR4 interaction. (a) Mouse NSCs (nestin+) that migrate chain-like toward ischemic brain explants express CXCR4. The phase image is shown under fluorescence microscopy below it, dual immunostained for nestin (green) and CXCR4 (red). (b) The area demarcated by the box in a, near the ischemic explant (asterisk), the border of which is indicated by the dotted line, is magnified where the nestin+ NSCs (green) are noted to coexpress CXCR4 (red); dual-immunoreactive cells seen as yellow in merged image. (c Left) Minimal chain migration of nestin+ cells to the contralateral noninjured explant correlating with an absence of SDF-1α in the explant, preserved only in meninges (arrow). (c Right) Robust migration of nestin+ NSCs toward injured explant with an increase in polarization and number of chains (see Figs. 6 and 7). (d) Neurospheres confronted with explants (asterisk) from normal control hemispheres show no migration (Left), neurospheres confronted at the same distance with explants from an ischemic hemisphere (asterisk) elaborate processes containing chains of migrating NSCs directed toward the explants (arrows) (Center); these behaviors are abrogated in neurospheres confronted with an ischemic explant (asterisk) but treated with a purified blocking antibody to CXCR4 (10 μg per explant) (Right). (e) Quantification of the percent of neurospheres with directed migration toward the explants. (f) Quantification of the formation of migratory chains toward the following explants: control, ischemic, or ischemic treated with anti-CXCR4 antibody. The latter condition reduces the number of migratory chains (P < 0.001).
Fig. 5.
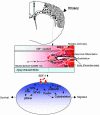
Model of inflammation-directed homing of NSCs toward pathology (as modeled by SDF-1α–CXCR4 interaction). As a result of injury, surviving or invading glia, microglia, and endothelial cells, the first responders, may produce chemoattractants (e.g., SDF-1α) that direct NSCs toward the ischemic core or penumbra (shaded area). NSCs of exogenous or endogenous origin (arrows), by virtue of their expression of chemokine receptors (e.g., CXCR4), respond to the chemokines that trigger the activation and phosphorylation of scaffold and adapter molecules within the NSCs and direct NSC migration in a chain-like fashion toward the source of the chemokines, allowing the NSCs to home to the pathology, to produce antiinflammatory/antiscarring molecules, and to engage local transiently expressed injury-induced signals.
Similar articles
- Hypoxia enhances CXCR4 expression favoring microglia migration via HIF-1alpha activation.
Wang X, Li C, Chen Y, Hao Y, Zhou W, Chen C, Yu Z. Wang X, et al. Biochem Biophys Res Commun. 2008 Jun 27;371(2):283-8. doi: 10.1016/j.bbrc.2008.04.055. Epub 2008 Apr 22. Biochem Biophys Res Commun. 2008. PMID: 18435916 - Physical exercise regulates neural stem cells proliferation and migration via SDF-1α/CXCR4 pathway in rats after ischemic stroke.
Luo J, Hu X, Zhang L, Li L, Zheng H, Li M, Zhang Q. Luo J, et al. Neurosci Lett. 2014 Aug 22;578:203-8. doi: 10.1016/j.neulet.2014.06.059. Epub 2014 Jul 7. Neurosci Lett. 2014. PMID: 25010020 - Genetically manipulated progenitor/stem cells restore function to the infarcted heart via the SDF-1α/CXCR4 signaling pathway.
Wang Y, Luther K. Wang Y, et al. Prog Mol Biol Transl Sci. 2012;111:265-84. doi: 10.1016/B978-0-12-398459-3.00012-5. Prog Mol Biol Transl Sci. 2012. PMID: 22917235 Review. - Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis.
Kucia M, Reca R, Miekus K, Wanzeck J, Wojakowski W, Janowska-Wieczorek A, Ratajczak J, Ratajczak MZ. Kucia M, et al. Stem Cells. 2005 Aug;23(7):879-94. doi: 10.1634/stemcells.2004-0342. Epub 2005 May 11. Stem Cells. 2005. PMID: 15888687 Review.
Cited by
- The immune system and developmental programming of brain and behavior.
Bilbo SD, Schwarz JM. Bilbo SD, et al. Front Neuroendocrinol. 2012 Aug;33(3):267-86. doi: 10.1016/j.yfrne.2012.08.006. Epub 2012 Sep 9. Front Neuroendocrinol. 2012. PMID: 22982535 Free PMC article. Review. - The roles of hypoxia-inducible factors in regulating neural stem cells migration to glioma stem cells and determinating their fates.
Zhang S, Luo X, Wan F, Lei T. Zhang S, et al. Neurochem Res. 2012 Dec;37(12):2659-66. doi: 10.1007/s11064-012-0879-x. Epub 2012 Sep 19. Neurochem Res. 2012. PMID: 22991140 Review. - Isolation and characterisation of mesenchymal stem cells derived from human placenta tissue.
Vellasamy S, Sandrasaigaran P, Vidyadaran S, George E, Ramasamy R. Vellasamy S, et al. World J Stem Cells. 2012 Jun 26;4(6):53-61. doi: 10.4252/wjsc.v4.i6.53. World J Stem Cells. 2012. PMID: 22993662 Free PMC article. - Stem cell therapy for the spinal cord.
Donnelly EM, Lamanna J, Boulis NM. Donnelly EM, et al. Stem Cell Res Ther. 2012 Jul 9;3(4):24. doi: 10.1186/scrt115. Stem Cell Res Ther. 2012. PMID: 22776143 Free PMC article. Review. - SDF-1 activates papillary label-retaining cells during kidney repair from injury.
Oliver JA, Maarouf O, Cheema FH, Liu C, Zhang QY, Kraus C, Zeeshan Afzal M, Firdous M, Klinakis A, Efstratiadis A, Al-Awqati Q. Oliver JA, et al. Am J Physiol Renal Physiol. 2012 Jun 1;302(11):F1362-73. doi: 10.1152/ajprenal.00202.2011. Epub 2012 Mar 28. Am J Physiol Renal Physiol. 2012. PMID: 22461304 Free PMC article.
References
- Imitola, J., Snyder, E. Y. & Khoury, S. J. (2003) Physiol. Genomics 14, 171–197. - PubMed
- Park, K. I., Ourednik, J., Ourednik, V., Taylor, R. M., Aboody, K. S., Auguste, K. I., Lachyankar, M. B., Redmond, D. E. & Snyder, E. Y. (2002) Gene Ther. 9, 613–624. - PubMed
- Park, K. I., Teng, Y. D. & Snyder, E. Y. (2002) Nat. Biotechnol. 20, 1111–1117. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials