NAADP binding to its target protein in sea urchin eggs requires phospholipids - PubMed (original) (raw)
NAADP binding to its target protein in sea urchin eggs requires phospholipids
Dev Churamani et al. Biochem J. 2005.
Abstract
Mobilization of intracellular Ca2+ pools by NAADP (nicotinic acid-adenine dinucleotide phosphate) is becoming increasingly recognized as an important determinant of complex Ca2+ signals. However, the properties of the putative Ca2+ channel activated by NAADP are poorly defined. In the present study, we provide evidence that binding of NAADP to its target protein in sea urchin eggs requires phospholipids. Decreasing the level of protein-bound lipid in detergent extracts by either dilution of the preparation at a fixed detergent concentration or increasing the detergent concentration at a fixed protein concentration inhibited [32P]NAADP binding. These effects were prevented by the addition of phospholipids, but not other related molecules, were reversible and were associated with a marked decrease in the apparent affinity of the target protein for its ligand. Additionally, we show that the extent of dissociation of NAADP-receptor ligand complexes during gel filtration in the presence of detergent correlates well with the extent of delipidation. Our data highlight the importance of the lipid environment for interaction of NAADP with its target protein.
Figures
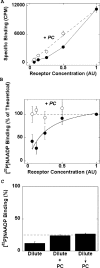
Figure 1. Effect of dilution at a fixed detergent concentration on [32P]NAADP binding to solubilized sea urchin egg homogenates
(A) Non-linear binding of [32P]NAADP to diluted solubilized egg homogenates. Typical experiment (performed in triplicate; means±S.D.) showing specific binding of a saturating concentration of [32P]NAADP (0.5–1 nM) to varying concentrations of egg homogenates solubilized with Triton X-100 following a 10-fold dilution of the preparation in detergent-free binding medium (final Triton X-100 concentration=0.1%, w/v). Solubilized preparations were used directly for radioligand binding or first serially diluted (2–16-fold) in binding medium+1% (w/v) Triton X-100 with (○) or without (●) 2.5 mg/ml PC, to yield arbitrary receptor concentrations of 1 and 0.5–0.125 respectively. Results from several experiments of this type (_n_=3) are shown in (B), where data are expressed as a percentage of that expected, assuming a linear relationship between receptor concentration and [32P]NAADP binding (broken line). (C) Inhibitory effects of dilution on [32P]NAADP binding are reversible. Binding of [32P]NAADP to soluble preparations that had been diluted 4-fold (receptor concentration=0.25) with Triton X-100 (1%, w/v) either in the absence (Dilute) or presence of PC added simultaneously (Dilute+PC) or 5 min after dilution (Dilute→PC). See the Materials and methods section for further details. Data are normalized to binding to undiluted samples (receptor concentration=1). AU, arbitrary units.
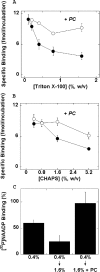
Figure 2. Effect of detergent concentration at a fixed dilution on [32P]NAADP binding to solubilized sea urchin egg homogenates
(A) Triton X-100 inhibits [32P]NAADP binding. Binding of [32P]NAADP to Triton X-100-solubilized extracts (arbitrary receptor concentration=1) was determined in the presence of Triton X-100 at the indicated concentrations in either the absence (●) or presence of PC (○). Results are from at least four independent experiments. (B) Similar experiments using CHAPS-solubilized samples. (C) Inhibitory effects of Triton X-100 on [32P]NAADP binding are reversible. Binding of [32P]NAADP was examined to Triton X-100-solubilized samples adjusted to a submaximal inhibitory concentration of detergent (0.4%, w/v) and compared with samples where further detergent was added (after a 5 min incubation) to a final concentration of 1.6% (w/v) in either the absence (0.4%→1.6%) or presence of 4 mg/ml PC (0.4%→1.6 %+PC). Data are normalized to [32P]NAADP binding at 0.1% (w/v) Triton X-100.
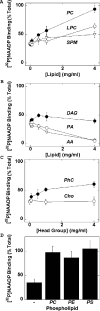
Figure 3. Inhibitory effects of delipidation on [32P]NAADP binding are specifically prevented by phospholipids
(A–C) Lack of effect of phospholipid-related molecules on detergent-mediated inhibition of [32P]NAADP binding. Binding of [32P]NAADP to solubilized samples was determined in the presence of 1.6% (w/v) Triton X-100 and the indicated concentration of test compound. (A) Effect of PC (●), LPC (○) and SPM (∇). (B) Effect of diacylglycerol (DAG, ●) and two unsaturated fatty acids, PA (○) and AA (∇). (C) Effect of the head group constituents phosphocholine (PhC, ●) and choline (Cho, ○). (D) Other phospholipids prevent detergent-mediated inhibition of [32P]NAADP binding. Similar experiments to (A–C) using PC, PS or PE (4 mg/ml), or no phospholipid (−). All data (from at least three experiments) are expressed relative to binding of [32P]NAADP determined at 0.1% (w/v) Triton X-100.
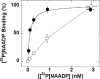
Figure 4. Inhibitory effects of delipidation on [32P]NAADP binding are due to a decrease in the apparent affinity of the target protein for its ligand
Binding of various concentrations of [32P]NAADP to Triton X-100-solubilized preparations at a final detergent concentration of 0.1% (●) or 1.6% (○). Data are from at least three experiments.
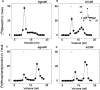
Figure 5. Delipidation of solubilized sea urchin egg homogenates by gel filtration
Triton X-100-solubilized sea urchin egg homogenates labelled with [32P]NAADP before solubilization (A and B) or [3H]PC after solubilization (C and D) were separated by gel filtration in KGluIM (A and C) or KClIM (B and D), and radioactivity of the collected fractions was determined. Both media were supplemented with 1% (w/v) Triton X-100. The inset in (B) shows the distribution of recovered radioactivity following precipitation of the collected fractions with poly(ethylene glycol). The triangle represents the elution of apoferritin (molecular mass of 443 kDa). Data are from at least three experiments.
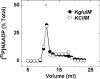
Figure 6. Gel-filtration analysis of solubilized sea urchin egg homogenates in the presence of PC
[32P]NAADP-labelled sea urchin egg homogenates solubilized with Triton X-100 were separated by gel filtration in KGluIM (●) or KClIM (○) as in Figure 5, except that PC (1 mg/ml) was included in the gel filtration medium. Data are from three experiments.
Similar articles
- Modulation of NAADP (nicotinic acid-adenine dinucleotide phosphate) receptors by K+ ions: evidence for multiple NAADP receptor conformations.
Dickinson GD, Patel S. Dickinson GD, et al. Biochem J. 2003 Nov 1;375(Pt 3):805-12. doi: 10.1042/BJ20030672. Biochem J. 2003. PMID: 12914540 Free PMC article. - Unique kinetics of nicotinic acid-adenine dinucleotide phosphate (NAADP) binding enhance the sensitivity of NAADP receptors for their ligand.
Patel S, Churchill GC, Galione A. Patel S, et al. Biochem J. 2000 Dec 15;352 Pt 3(Pt 3):725-9. Biochem J. 2000. PMID: 11104679 Free PMC article. - Characterization of NAADP(+) binding in sea urchin eggs.
Billington RA, Genazzani AA. Billington RA, et al. Biochem Biophys Res Commun. 2000 Sep 16;276(1):112-6. doi: 10.1006/bbrc.2000.3444. Biochem Biophys Res Commun. 2000. PMID: 11006092 - NAADP receptors.
Galione A, Ruas M. Galione A, et al. Cell Calcium. 2005 Sep-Oct;38(3-4):273-80. doi: 10.1016/j.ceca.2005.06.031. Cell Calcium. 2005. PMID: 16111747 Review. - The NAADP receptor: commentary on Billington et al.
Galione A, Parrington J, Dowden J. Galione A, et al. Br J Pharmacol. 2004 Aug;142(8):1203-7. doi: 10.1038/sj.bjp.0705888. Epub 2004 Jul 20. Br J Pharmacol. 2004. PMID: 15265809 Free PMC article. Review.
Cited by
- Time sensing by NAADP receptors.
Churamani D, Dickinson GD, Ziegler M, Patel S. Churamani D, et al. Biochem J. 2006 Jul 15;397(2):313-20. doi: 10.1042/BJ20060179. Biochem J. 2006. PMID: 16551267 Free PMC article. - The two-pore channel TPCN2 mediates NAADP-dependent Ca(2+)-release from lysosomal stores.
Zong X, Schieder M, Cuny H, Fenske S, Gruner C, Rötzer K, Griesbeck O, Harz H, Biel M, Wahl-Schott C. Zong X, et al. Pflugers Arch. 2009 Sep;458(5):891-9. doi: 10.1007/s00424-009-0690-y. Epub 2009 Jun 26. Pflugers Arch. 2009. PMID: 19557428 Free PMC article. - The Molecular Basis for Ca2+ Signalling by NAADP: Two-Pore Channels in a Complex?
Marchant JS, Lin-Moshier Y, Walseth TF, Patel S. Marchant JS, et al. Messenger (Los Angel). 2012 Jun 1;1(1):63-76. doi: 10.1166/msr.2012.1003. Messenger (Los Angel). 2012. PMID: 25309835 Free PMC article.
References
- Berridge M. J., Lipp P., Bootman M. D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000;1:11–21. - PubMed
- Taylor C. W. Inositol trisphosphate receptors: Ca2+-modulated intracellular Ca2+ release channels. Biochim. Biophys. Acta. 1998;1436:19–33. - PubMed
- Patel S., Joseph S. K., Thomas A. P. Molecular properties of inositol 1,4,5-trisphosphate receptors. Cell Calcium. 1999;25:247–264. - PubMed
- Fill M., Copello J. A. Ryanodine receptor calcium release channels. Physiol. Rev. 2002;82:893–922. - PubMed
- Lee H. C. Physiological functions of cyclic ADP-ribose and NAADP as calcium messengers. Annu. Rev. Pharmacol. Toxicol. 2001;41:317–345. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous