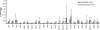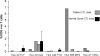Cytotoxic T lymphocyte therapy for Epstein-Barr virus+ Hodgkin's disease - PubMed (original) (raw)
. 2004 Dec 20;200(12):1623-33.
doi: 10.1084/jem.20040890.
Laura Aguilar, Karin C Straathof, Benedikt Gahn, M Helen Huls, Alexandra Rousseau, John Sixbey, M Victoria Gresik, George Carrum, Melissa Hudson, Dagmar Dilloo, Adrian Gee, Malcolm K Brenner, Cliona M Rooney, Helen E Heslop
Affiliations
- PMID: 15611290
- PMCID: PMC2211993
- DOI: 10.1084/jem.20040890
Cytotoxic T lymphocyte therapy for Epstein-Barr virus+ Hodgkin's disease
Catherine M Bollard et al. J Exp Med. 2004.
Abstract
Epstein Barr virus (EBV)+ Hodgkin's disease (HD) expresses clearly identified tumor antigens derived from the virus and could, in principle, be a target for adoptive immunotherapy with viral antigen-specific T cells. However, like most tumor-associated antigens in immunocompetent hosts, these potential targets are only weakly immunogenic, consisting primarily of the latent membrane protein (LMP)1 and LMP2 antigens. Moreover, Hodgkin tumors possess a range of tumor evasion strategies. Therefore, the likely value of immunotherapy with EBV-specific cytotoxic effector cells has been questioned. We have now used a combination of gene marking, tetramer, and functional analyses to track the fate and assess the activity of EBV cytotoxic T lymphocyte (CTL) lines administered to 14 patients treated for relapsed EBV+ HD. Gene marking studies showed that infused effector cells could further expand by several logs in vivo, contribute to the memory pool (persisting up to 12 mo), and traffic to tumor sites. Tetramer and functional analyses showed that T cells reactive with the tumor-associated antigen LMP2 were present in the infused lines, expanded in peripheral blood after infusion, and also entered tumor. Viral load decreased, demonstrating the biologic activity of the infused CTLs. Clinically, EBV CTLs were well tolerated, could control type B symptoms (fever, night sweats, and weight loss), and had antitumor activity. After CTL infusion, five patients were in complete remission at up to 40 mo, two of whom had clearly measurable tumor at the time of treatment. One additional patient had a partial response, and five had stable disease. The performance and fate of these human tumor antigen-specific T cells in vivo suggests that they might be of value for the treatment of EBV+ Hodgkin lymphoma.
Figures
Figure 1.
All CTL lines generated were polyclonal with unique Vβ repertoires. To analyze the Vβ repertoires of the CTL lines generated from six patients (gray) and six normal donors (shaded), the CTLs were stained with anti-CD3 and 24 Vβ antibodies grouped into 8 vials. Surface immunofluorescence was analyzed by flow cytometry.
Figure 2.
EBV-specific CTL lines derived from Hodgkin patients and normal donors contained LMP2-specific T cell populations on tetramer analysis. The frequency of LMP2-specific T cells in CTL lines generated from 11 patients with relapsed HD were compared with CTL lines from 7 normal donors with relevant HLA alleles. CTLs were costained with PE-conjugated tetramers and FITC-conjugated CD8 and PerCP-conjugated CD3. Tetramers used to test the lines were as follows: HLA-A02*01-CLGGLLTMV, HLA-A02*01-FLYALALLL, HLA-A11*01-SSCSSCPLSKI, HLA-A23*01-PYLFWLAAI, HLA-A24-TYGPVFMSL, and HLA-B35*01-MGSLEMVPM. The average (± SD) of the results obtained from the normal donor lines are shown in black compared to the results obtained from each Hodgkin line tested, which are shown in gray.
Figure 3.
Infused CTLs gene marked with the neomycin resistance gene can persist in vivo for up to 12 mo. We used Q-PCR to quantify the presence of the marker gene DNA in PBMCs after CTL infusion in the patients who received gene-marked cells. Control DNA was prepared by diluting G1Na-transduced K-562 cells (one integrant per cell) with nontransduced K-562 to give mixtures containing 0.01–10% neo+ cells. Therefore, results are reported as a percent of neo+ cells in PBMCs after infusion.
Figure 4.
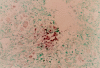
Infused CTLs can home to tumor sites. In situ PCR analysis of a mediastinal Hodgkin tumor obtained at autopsy 2 mo after receiving gene-marked EBV-specific CTLs demonstrates gene-marked, tumor-infiltrating lymphocytes.
Figure 5.
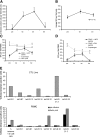
The frequency of EBV- and LMP2-specific T cells increase after infusion of polyclonal EBV-specific CTLs. (A) Tetramer analysis was used to compare the frequency of LMP2-specific CTLs before and after infusion in the four patients who were HLA-A02*01. The mean number ± SD of A02*01 tetramer+ cells per 106 CD8+ T cells are shown before and after infusion. (B) In six patients where tetramer reagents were not available or where sample volumes were small, ELISPOT analysis was used to compare the mean frequency of peripheral blood T cells (± standard error) responding to LCLs by IFN-γ secretion before and after CTL infusion. (C) In patients 11 and (D) 12 where the HLA-restricted LMP2 peptide was known, peripheral blood T cells were incubated with LMP2 peptide–pulsed PHA blasts (black diamond) or CMV lysate/PHA blasts pulsed with CMV peptide (gray triangle) or superantigen (gray square). The number of IFN-γ SFCs per 106 mononuclear cells was measured. The average IFN-γ SFCs ± standard error in response to the B35-restricted LMP2 peptide MGS before and after CTL infusion in patient 11 are shown in C, whereas the LMP2 peptide+ populations before and after CTL measured with the HLA-A11–restricted LMP2 peptide SSC in patient 12 are shown in D. (E) The Vβ phenotype of the PE-labeled LMP2 tetramer+ T cells from patient 11 were analyzed by flow cytometry using available FITC-labeled Vβ antibodies in the infused CTL line (black stripes) and were compared to the phenotype of peripheral blood LMP2 tetramer+ cells obtained before (black bars) and 6 wk (gray bars) after CTL infusion. (F) In the patient who had a partial response (patient 3), gene-marked cells were detected in the pleural effusion at the level of 0.65% using Q-PCR. A sample of this patient's pleural fluid was also tested for the presence of LMP2-specific CTLs using the HLA-A02*01 tetramer CLG. As shown in F, 1.21% of the patient's CTL line was positive for this tetramer, and these LMP2 tetramer–specific CTLs were detected in the pleural fluid at a level three times higher than that in the peripheral blood. (G) The EBV DNA levels as determined by Q-PCR for patients 11 (black) and 12 (gray).
Figure 5.
The frequency of EBV- and LMP2-specific T cells increase after infusion of polyclonal EBV-specific CTLs. (A) Tetramer analysis was used to compare the frequency of LMP2-specific CTLs before and after infusion in the four patients who were HLA-A02*01. The mean number ± SD of A02*01 tetramer+ cells per 106 CD8+ T cells are shown before and after infusion. (B) In six patients where tetramer reagents were not available or where sample volumes were small, ELISPOT analysis was used to compare the mean frequency of peripheral blood T cells (± standard error) responding to LCLs by IFN-γ secretion before and after CTL infusion. (C) In patients 11 and (D) 12 where the HLA-restricted LMP2 peptide was known, peripheral blood T cells were incubated with LMP2 peptide–pulsed PHA blasts (black diamond) or CMV lysate/PHA blasts pulsed with CMV peptide (gray triangle) or superantigen (gray square). The number of IFN-γ SFCs per 106 mononuclear cells was measured. The average IFN-γ SFCs ± standard error in response to the B35-restricted LMP2 peptide MGS before and after CTL infusion in patient 11 are shown in C, whereas the LMP2 peptide+ populations before and after CTL measured with the HLA-A11–restricted LMP2 peptide SSC in patient 12 are shown in D. (E) The Vβ phenotype of the PE-labeled LMP2 tetramer+ T cells from patient 11 were analyzed by flow cytometry using available FITC-labeled Vβ antibodies in the infused CTL line (black stripes) and were compared to the phenotype of peripheral blood LMP2 tetramer+ cells obtained before (black bars) and 6 wk (gray bars) after CTL infusion. (F) In the patient who had a partial response (patient 3), gene-marked cells were detected in the pleural effusion at the level of 0.65% using Q-PCR. A sample of this patient's pleural fluid was also tested for the presence of LMP2-specific CTLs using the HLA-A02*01 tetramer CLG. As shown in F, 1.21% of the patient's CTL line was positive for this tetramer, and these LMP2 tetramer–specific CTLs were detected in the pleural fluid at a level three times higher than that in the peripheral blood. (G) The EBV DNA levels as determined by Q-PCR for patients 11 (black) and 12 (gray).
Figure 5.
The frequency of EBV- and LMP2-specific T cells increase after infusion of polyclonal EBV-specific CTLs. (A) Tetramer analysis was used to compare the frequency of LMP2-specific CTLs before and after infusion in the four patients who were HLA-A02*01. The mean number ± SD of A02*01 tetramer+ cells per 106 CD8+ T cells are shown before and after infusion. (B) In six patients where tetramer reagents were not available or where sample volumes were small, ELISPOT analysis was used to compare the mean frequency of peripheral blood T cells (± standard error) responding to LCLs by IFN-γ secretion before and after CTL infusion. (C) In patients 11 and (D) 12 where the HLA-restricted LMP2 peptide was known, peripheral blood T cells were incubated with LMP2 peptide–pulsed PHA blasts (black diamond) or CMV lysate/PHA blasts pulsed with CMV peptide (gray triangle) or superantigen (gray square). The number of IFN-γ SFCs per 106 mononuclear cells was measured. The average IFN-γ SFCs ± standard error in response to the B35-restricted LMP2 peptide MGS before and after CTL infusion in patient 11 are shown in C, whereas the LMP2 peptide+ populations before and after CTL measured with the HLA-A11–restricted LMP2 peptide SSC in patient 12 are shown in D. (E) The Vβ phenotype of the PE-labeled LMP2 tetramer+ T cells from patient 11 were analyzed by flow cytometry using available FITC-labeled Vβ antibodies in the infused CTL line (black stripes) and were compared to the phenotype of peripheral blood LMP2 tetramer+ cells obtained before (black bars) and 6 wk (gray bars) after CTL infusion. (F) In the patient who had a partial response (patient 3), gene-marked cells were detected in the pleural effusion at the level of 0.65% using Q-PCR. A sample of this patient's pleural fluid was also tested for the presence of LMP2-specific CTLs using the HLA-A02*01 tetramer CLG. As shown in F, 1.21% of the patient's CTL line was positive for this tetramer, and these LMP2 tetramer–specific CTLs were detected in the pleural fluid at a level three times higher than that in the peripheral blood. (G) The EBV DNA levels as determined by Q-PCR for patients 11 (black) and 12 (gray).
Figure 6.
Tumor responses. (A) In patient 14 whose cervical lymph nodes responded to infusion of CTLs that were not genetically marked, immunohistochemical analysis revealed CD8+ tumor-infiltrating lymphocytes only after CTL infusion. This corresponded to the disappearance of LMP1+ cells. (B) A gallium scan demonstrating increased hilar uptake is observed in patient 12 3 mo after autologous SCT. The follow-up scan 6 wk after CTL infusion is reported as normal. (C) To demonstrate LMP2 specificity within patient 12's infused CTL line in vitro, the frequency of CTLs specific for the HLA-A11–restricted LMP2 epitope SSC was assessed using tetramer and (D) ELISPOT analysis, the latter using SSC peptide–pulsed PHA blasts as targets. (E) Further, the percent-specific 51Cr release was determined 6 h after coincubation with HLA-A11–matched fibroblasts transduced with Ad5LMP2 (gray triangle) or Ad5GFP (gray square) and autologous LCLs (black diamond). The percent-specific lysis at the indicated effector/target ratios for the EBV CTL line from patient 12 is shown.
Figure 6.
Tumor responses. (A) In patient 14 whose cervical lymph nodes responded to infusion of CTLs that were not genetically marked, immunohistochemical analysis revealed CD8+ tumor-infiltrating lymphocytes only after CTL infusion. This corresponded to the disappearance of LMP1+ cells. (B) A gallium scan demonstrating increased hilar uptake is observed in patient 12 3 mo after autologous SCT. The follow-up scan 6 wk after CTL infusion is reported as normal. (C) To demonstrate LMP2 specificity within patient 12's infused CTL line in vitro, the frequency of CTLs specific for the HLA-A11–restricted LMP2 epitope SSC was assessed using tetramer and (D) ELISPOT analysis, the latter using SSC peptide–pulsed PHA blasts as targets. (E) Further, the percent-specific 51Cr release was determined 6 h after coincubation with HLA-A11–matched fibroblasts transduced with Ad5LMP2 (gray triangle) or Ad5GFP (gray square) and autologous LCLs (black diamond). The percent-specific lysis at the indicated effector/target ratios for the EBV CTL line from patient 12 is shown.
Similar articles
- Epstein-Barr virus-specific cytotoxic T lymphocyte responses in the blood and tumor site of Hodgkin's disease patients: implications for a T-cell-based therapy.
Chapman AL, Rickinson AB, Thomas WA, Jarrett RF, Crocker J, Lee SP. Chapman AL, et al. Cancer Res. 2001 Aug 15;61(16):6219-26. Cancer Res. 2001. PMID: 11507075 - In vivo expansion of LMP 1- and 2-specific T-cells in a patient who received donor-derived EBV-specific T-cells after allogeneic stem cell transplantation.
Bollard CM, Gottschalk S, Huls MH, Molldrem J, Przepiorka D, Rooney CM, Heslop HE. Bollard CM, et al. Leuk Lymphoma. 2006 May;47(5):837-42. doi: 10.1080/10428190600604724. Leuk Lymphoma. 2006. PMID: 16753867 - Epstein-Barr virus (EBV)-specific cytotoxic T lymphocytes for the treatment of patients with EBV-positive relapsed Hodgkin's disease.
Roskrow MA, Suzuki N, Gan Yj, Sixbey JW, Ng CY, Kimbrough S, Hudson M, Brenner MK, Heslop HE, Rooney CM. Roskrow MA, et al. Blood. 1998 Apr 15;91(8):2925-34. Blood. 1998. PMID: 9531603 Clinical Trial. - Immunotherapy for Epstein-Barr virus-associated cancers in children.
Straathof KC, Bollard CM, Rooney CM, Heslop HE. Straathof KC, et al. Oncologist. 2003;8(1):83-98. doi: 10.1634/theoncologist.8-1-83. Oncologist. 2003. PMID: 12604735 Review. - Adoptive immunotherapy for Hodgkin's lymphoma.
Kennedy-Nasser AA, Bollard CM, Rooney CM. Kennedy-Nasser AA, et al. Int J Hematol. 2006 Jun;83(5):385-90. doi: 10.1532/IJH97.06107. Int J Hematol. 2006. PMID: 16787867 Review.
Cited by
- C-C chemokine receptor type-4 transduction of T cells enhances interaction with dendritic cells, tumor infiltration and therapeutic efficacy of adoptive T cell transfer.
Rapp M, Grassmann S, Chaloupka M, Layritz P, Kruger S, Ormanns S, Rataj F, Janssen KP, Endres S, Anz D, Kobold S. Rapp M, et al. Oncoimmunology. 2015 Oct 29;5(3):e1105428. doi: 10.1080/2162402X.2015.1105428. eCollection 2016 Mar. Oncoimmunology. 2015. PMID: 27195186 Free PMC article. - Epstein Barr virus-associated lymphoproliferative diseases: the virus as a therapeutic target.
Tse E, Kwong YL. Tse E, et al. Exp Mol Med. 2015 Jan 23;47(1):e136. doi: 10.1038/emm.2014.102. Exp Mol Med. 2015. PMID: 25613733 Free PMC article. Review. - Antigen-specific T-cell memory is preserved in children treated for acute lymphoblastic leukemia.
Haining WN, Neuberg DS, Keczkemethy HL, Evans JW, Rivoli S, Gelman R, Rosenblatt HM, Shearer WT, Guenaga J, Douek DC, Silverman LB, Sallan SE, Guinan EC, Nadler LM. Haining WN, et al. Blood. 2005 Sep 1;106(5):1749-54. doi: 10.1182/blood-2005-03-1082. Epub 2005 May 26. Blood. 2005. PMID: 15920008 Free PMC article. Clinical Trial. - Immunotherapies for Hodgkin's lymphoma.
Kasamon YL, Ambinder RF. Kasamon YL, et al. Crit Rev Oncol Hematol. 2008 May;66(2):135-44. doi: 10.1016/j.critrevonc.2007.10.001. Epub 2007 Nov 19. Crit Rev Oncol Hematol. 2008. PMID: 18023356 Free PMC article. Review. - T-cell engager-armed oncolytic vaccinia virus significantly enhances antitumor therapy.
Yu F, Wang X, Guo ZS, Bartlett DL, Gottschalk SM, Song XT. Yu F, et al. Mol Ther. 2014 Jan;22(1):102-11. doi: 10.1038/mt.2013.240. Epub 2013 Oct 17. Mol Ther. 2014. PMID: 24135899 Free PMC article.
References
- Rooney, C.M., C.A. Smith, C. Ng, S.K. Loftin, C. Li, R.A. Krance, M.K. Brenner, and H.E. Heslop. 1995. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr virus-related lymphoproliferation. Lancet. 345:9–13. - PubMed
- Heslop, H.E., C.Y.C. Ng, C. Li, C.A. Smith, S.K. Loftin, R.A. Krance, M.K. Brenner, and C.M. Rooney. 1996. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat. Med. 2:551–555. - PubMed
- Rooney, C.M., C.A. Smith, C.Y.C. Ng, S.K. Loftin, J.W. Sixbey, Y.-J. Gan, D.-K. Srivastava, L.C. Bowman, R.A. Krance, M.K. Brenner, and H.E. Heslop. 1998. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 92:1549–1555. - PubMed
- Gustafsson, A., V. Levitsky, J.Z. Zou, T. Frisan, T. Dalianis, P. Ljungman, O. Ringden, J. Winiarski, I. Ernberg, and M.G. Masucci. 2000. Epstein-Barr virus (EBV) load in bone marrow transplant recipients at risk to develop posttransplant lymphoproliferative disease: prophylactic infusion of EBV-specific cytotoxic T cells. Blood. 95:807–814. - PubMed
- O'Reilly, R.J., T.N. Small, E. Papadopoulos, K. Lucas, J. Lacerda, and L. Koulova. 1998. Adoptive immunotherapy for Epstein-Barr virus-associated lymphoproliferative disorders complicating marrow allografts. Springer Semin. Immunopathol. 20:455–491. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K01 RR000188/RR/NCRR NIH HHS/United States
- M01 RR000188/RR/NCRR NIH HHS/United States
- RR00188/RR/NCRR NIH HHS/United States
- P01 CA094237/CA/NCI NIH HHS/United States
- P01 CA94237/CA/NCI NIH HHS/United States
- R01 CA74126/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases