Role of Dok-1 and Dok-2 in myeloid homeostasis and suppression of leukemia - PubMed (original) (raw)
. 2004 Dec 20;200(12):1681-7.
doi: 10.1084/jem.20041247.
Masaki Shirakata, Atsushi Iwama, Asuka Ishii, Yasuhiro Ebihara, Mitsujiro Osawa, Kazuho Honda, Hisaaki Shinohara, Katsuko Sudo, Kohichiro Tsuji, Hiromitsu Nakauchi, Yoichiro Iwakura, Hisamaru Hirai, Hideaki Oda, Tadashi Yamamoto, Yuji Yamanashi
Affiliations
- PMID: 15611294
- PMCID: PMC2211997
- DOI: 10.1084/jem.20041247
Role of Dok-1 and Dok-2 in myeloid homeostasis and suppression of leukemia
Tomoharu Yasuda et al. J Exp Med. 2004.
Abstract
Dok-1 and Dok-2 are closely related rasGAP-associated docking proteins expressed preferentially in hematopoietic cells. Although they are phosphorylated upon activation of many protein tyrosine kinases (PTKs), including those coupled with cytokine receptors and oncogenic PTKs like Bcr-Abl, their physiological roles are largely unidentified. Here, we generated mice lacking Dok-1 and/or Dok-2, which included the double-deficient mice succumbed to myeloproliferative disease resembling human chronic myelogenous leukemia (CML) and chronic myelomonocytic leukemia. The double-deficient mice displayed medullary and extramedullary hyperplasia of granulocyte/macrophage progenitors with leukemic potential, and their myeloid cells showed hyperproliferation and hypo-apoptosis upon treatment and deprivation of cytokines, respectively. Consistently, the mutant myeloid cells showed enhanced Erk and Akt activation upon cytokine stimulation. Moreover, loss of Dok-1 and/or Dok-2 induced blastic transformation of chronic phase CML-like disease in mice carrying the bcr-abl gene, a cause of CML. These findings demonstrate that Dok-1 and Dok-2 are key negative regulators of cytokine responses and are essential for myeloid homeostasis and suppression of leukemia.
Figures
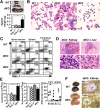
Figure 1.
dKO mice develop a myeloproliferative disease resembling human CML and CMMoL. (A) Massive enlargement of the spleen in dKO mice compared with wild-type controls at 13 mo of age (top). Spleen weights of wild-type (n = 25), Dok-1−/− (n = 10), Dok-2−/− (n = 17), and dKO (n = 12) mice measured at 13–15 mo of age (bottom). The mean value with the SE is demonstrated. (B) Cytospin specimens of splenocytes from dKO mice at 15 mo of age are characteristic of myeloproliferative disease with marked myeloid hyperplasia comprised of granulocytes/monocytes in various stages of differentiation, including atypical myeloid cells (arrowheads). (C) Flow cytometry for Mac-1 and Gr-1 on bone marrow, spleen, or peripheral blood cells from mice at 15 mo of age. (D) Mononuclear cell infiltration into perivascular areas in dKO kidney and liver. Higher magnification of each boxed area (bottom) shows the infiltrating neutrophils (arrowheads). (E) Leukocytes (left) or erythrocytes (middle) in peripheral blood of wild-type (n = 22), Dok-1−/− (n = 6), Dok-2−/− (n = 7), or dKO (n = 12) mice at 13–15 mo of age were enumerated, and mean values with each SE are demonstrated. Differential cell counts were determined (right) from peripheral blood smears of wild-type (○) or dKO (•) mice and are demonstrated with each mean value. Ne, neutrophil; Mo, monocyte; Eo, eosinophil; Ly, lymphocyte. (F) Gross appearance of enlarged dKO kidneys bearing histiocytic sarcoma or wild-type controls for each mouse at 15 mo of age (left). Histological sections of dKO kidney were stained for macrophage markers Mac-1 or F4/80 (right). Positively stained tumor cells surround unstained cells in the glomerulus (G) and uriniferous tubules (U). Statistical significance compared with wild-type was determined with the Student's t test. *, P < 0.01.
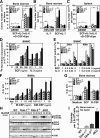
Figure 2.
Hyperplasia of granulocyte/macrophage progenitors in dKO mice and hyperactivation of their myeloid cells upon stimulation with cytokines. (A–C) Colony formation assays for CFU-GEMM (GEMM), CFU-GM (GM), or CFU-preB (preB) were performed with bone marrow or spleen cells of the mice at 4 mo of age in the presence of the indicated cytokine(s). Statistical significance compared with wild-type was determined with the Student's t test. *, P < 0.01; **, P < 0.001. (D and E) Proliferation and apoptosis of BMMCs were evaluated based on [3H]thymidine incorporation and annexin V binding, respectively, in the presence or absence of the indicated cytokine(s). Apoptotic rates were determined after the indicated culture period. (F) The proliferation of BMMφs was evaluated with the MTT assay after 5 d of culture in the presence of the indicated cytokine. (G) The proliferation of bone marrow cells from mice at 8 wk of age was evaluated with the MTT assay after 4 d of culture in the absence or presence (10 ng/ml) of the indicated cytokine. (A–G) Mean values and SEs are from triplicate experiments. (H) Activated Erk (p-Erk1/2), Akt (p-Akt), total Erk1/2, or Akt was examined with immunoblotting upon M-CSF treatment (10 ng/ml) of BMMφs. The position of each protein and period of stimulation are indicated. (I) Colony formation assay was performed with bone marrow cells of mice at 8 wk of age in the absence of cytokine. Mean values and SEs are from triplicate experiments.
Figure 3.
Cell autonomous progression of myeloproliferative disease in the absence of Dok-1 and Dok-2. Hematopoietic cells in lethally irradiated wild-type (Ly5.1 or Ly5.2 allotype) or dKO mice (Ly5.2) were reconstituted with bone marrow cells from 8-wk-old wild-type (Ly5.1 or Ly5.2) or dKO (Ly5.2) mice. Flow cytometry on peripheral blood cells of each recipient was performed at 9 mo after transplantation. The donor-derived myeloid cell compartment is circled in forward scatter (FSC) versus side scatter (SSC) parameters (top). Gr-1 and Mac-1 expression on donor-derived cells in peripheral blood of each recipient was also examined (bottom). Mice are with the C57BL/6 genetic background.
Figure 4.
Dok-1 and Dok-2 suppress the blastic transformation of the Bcr-Abl–induced CML-like disease. (A) Survival curves for Bcr-Abl mice lacking Dok-1 and/or Dok-2 and the nontransgenic controls. HH, HK, KH, and KK stand for mice with the dok-1 +/−:dok-2 +/−, dok-1 +/−: dok-2 −/−, dok-1 −/−: dok-2 +/−, and dok-1 −/−: dok-2 −/− loci, respectively, and T stands for mice carrying the bcr-abl transgene. For each genotype, 10 mice were monitored. Log rank statistical analysis was performed (*, P < 0.03, compared with the THH mice). (B) Tumors that developed in the lymph nodes (left, arrowheads) and thymus (right, arrow) of a TKK mouse (No. 4). (C) Lymphoblasts in tumors developed in lymph nodes of mice. (D) Surface expression of CD4 and CD8 on mononuclear cells in lymph nodes (LN), thymus (Thy), or peripheral blood (PB) of mice at 4 mo of age examined with flow cytometry. The HH mice were analyzed as an age-matched control. (E) Lymphoblasts in gastrointestinal lymphomas developed in large intestines of mice at 7 mo of age. The HH mouse was analyzed as an age-matched control.
Similar articles
- Role of Dok-1 and Dok-2 in leukemia suppression.
Niki M, Di Cristofano A, Zhao M, Honda H, Hirai H, Van Aelst L, Cordon-Cardo C, Pandolfi PP. Niki M, et al. J Exp Med. 2004 Dec 20;200(12):1689-95. doi: 10.1084/jem.20041306. J Exp Med. 2004. PMID: 15611295 Free PMC article. - Dok-1 and Dok-2 are negative regulators of lipopolysaccharide-induced signaling.
Shinohara H, Inoue A, Toyama-Sorimachi N, Nagai Y, Yasuda T, Suzuki H, Horai R, Iwakura Y, Yamamoto T, Karasuyama H, Miyake K, Yamanashi Y. Shinohara H, et al. J Exp Med. 2005 Feb 7;201(3):333-9. doi: 10.1084/jem.20041817. J Exp Med. 2005. PMID: 15699069 Free PMC article. - Dok-1 and Dok-2 are negative regulators of T cell receptor signaling.
Yasuda T, Bundo K, Hino A, Honda K, Inoue A, Shirakata M, Osawa M, Tamura T, Nariuchi H, Oda H, Yamamoto T, Yamanashi Y. Yasuda T, et al. Int Immunol. 2007 Apr;19(4):487-95. doi: 10.1093/intimm/dxm015. Epub 2007 Feb 27. Int Immunol. 2007. PMID: 17329234 - The roles of Dok family adapters in immunoreceptor signaling.
Mashima R, Hishida Y, Tezuka T, Yamanashi Y. Mashima R, et al. Immunol Rev. 2009 Nov;232(1):273-85. doi: 10.1111/j.1600-065X.2009.00844.x. Immunol Rev. 2009. PMID: 19909370 Review. - Modeling Philadelphia chromosome positive leukemias.
Wong S, Witte ON. Wong S, et al. Oncogene. 2001 Sep 10;20(40):5644-59. doi: 10.1038/sj.onc.1204638. Oncogene. 2001. PMID: 11607816 Review.
Cited by
- The non-genomic loss of function of tumor suppressors: an essential role in the pathogenesis of chronic myeloid leukemia chronic phase.
Crivellaro S, Carrà G, Panuzzo C, Taulli R, Guerrasio A, Saglio G, Morotti A. Crivellaro S, et al. BMC Cancer. 2016 May 16;16:314. doi: 10.1186/s12885-016-2346-6. BMC Cancer. 2016. PMID: 27184141 Free PMC article. Review. - Transcriptional regulation of the human tumor suppressor DOK1 by E2F1.
Siouda M, Yue J, Shukla R, Guillermier S, Herceg Z, Creveaux M, Accardi R, Tommasino M, Sylla BS. Siouda M, et al. Mol Cell Biol. 2012 Dec;32(23):4877-90. doi: 10.1128/MCB.01050-12. Epub 2012 Oct 1. Mol Cell Biol. 2012. PMID: 23028047 Free PMC article. - Plasma exosomal DOK3 reflects immunological states in lung tumor and predicts prognosis of gefitinib treatment.
Ochiai R, Hayashi K, Yamamoto H, Fujii R, Saichi N, Shinchi H, Ishida T, Honda T, Shimizu T, Matsutani N, Seki N, Kawamura M, Ueda K. Ochiai R, et al. Cancer Sci. 2022 Nov;113(11):3960-3971. doi: 10.1111/cas.15512. Epub 2022 Aug 26. Cancer Sci. 2022. PMID: 36028467 Free PMC article. - DOK6 promoter methylation serves as a potential biomarker affecting prognosis in de novo acute myeloid leukemia.
Sun GK, Tang LJ, Zhou JD, Xu ZJ, Yang L, Yuan Q, Ma JC, Liu XH, Lin J, Qian J, Yao DM. Sun GK, et al. Cancer Med. 2019 Oct;8(14):6393-6402. doi: 10.1002/cam4.2540. Epub 2019 Sep 4. Cancer Med. 2019. PMID: 31486300 Free PMC article.
References
- Carpino, N., D. Wisniewski, A. Strife, D. Marshak, R. Kobayashi, B. Stillman, and B. Clarkson. 1997. p62dok: a constitutively tyrosine-phosphorylated, GAP-associated protein in chronic myelogenous leukemia progenitor cells. Cell. 88:197–204. - PubMed
- Yamanashi, Y., and D. Baltimore. 1997. Identification of the Abl- and rasGAP-associated 62 kDa protein as a docking protein, Dok. Cell. 88:205–211. - PubMed
- Di Cristofano, A., N. Carpino, N. Dunant, G. Friedland, R. Kobayashi, A. Strife, D. Wisniewski, B. Clarkson, P.P. Pandolfi, and M.D. Resh. 1998. Molecular cloning and characterization of p56dok-2defines a new family of RasGAP-binding proteins. J. Biol. Chem. 273:4827–4830. - PubMed
- Jones, N., and D.J. Dumont. 1998. The Tek/Tie2 receptor signals through a novel Dok-related docking protein, Dok-R. Oncogene. 17:1097–1108. - PubMed
- Nelms, K., A.L. Snow, J. Hu-Li, and W.E. Paul. 1998. FRIP, a hematopoietic cell-specific rasGAP-interacting protein phosphorylated in response to cytokine stimulation. Immunity. 9:13–24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous