Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury - PubMed (original) (raw)
Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury
Sarah L Pull et al. Proc Natl Acad Sci U S A. 2005.
Abstract
We have identified cellular and molecular features of the stem cell niche required for marked amplification of mouse colonic epithelial progenitors (ColEPs) that occurs in response to wounding of the epithelium with dextran sodium sulfate. This regenerative response in areas adjacent to breaches in the epithelial barrier depends on the gut microbiota because ColEP proliferation is markedly diminished in germ-free animals. Analysis of conventionally raised C57BL/6 (B6) knockout mice lacking the Toll-like receptor signal transduction pathway component Myd88 and wild-type animals transplanted with Myd88(-/-) bone marrow, revealed that Myd88-mediated signaling through mesenchymal cells is also required for the ColEP response. Studies of B6 Csf1(op/op) (lacking macrophages) mice, Rag1(-/-) mice, and wild-type mice treated with neutrophil-specific Gr1 mAbs, disclosed that macrophages but not lymphocytes or neutrophils are necessary. GeneChip analysis of laser-capture-microdissected mesenchymal cells coupled with immunohistochemical and electron microscopic studies showed that, during the regenerative response, macrophages in the pericryptal stem cell niche express genes associated with their activation and extend processes to directly contact ColEPs near the crypt base. GeneChip analysis also identified a number of potential molecular mediators of regeneration expressed in the pericryptal progenitor niche, including secreted factors that stimulate epithelial proliferation and proteins involved in extracellular matrix and basement membrane function, stability, and growth factor binding. Together, these studies indicate that the colonic epithelial progenitor niche is a dynamic structure in which macrophages function as mobile "cellular transceivers" that coordinate inputs from luminal microbes and injured epithelium and transmit regenerative signals to neighboring ColEPs.
Figures
Fig. 1.
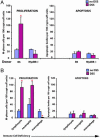
The intestinal microbiota and Myd88 signaling are necessary for the proper response to epithelial injury. (A_–_F)H+E-stained sections of descending colon from adult B6 mice. DSS induces focal ulcer formation (dashed boxes). (A and B) Wild-type CONV-R (i.e., acquired a microbiota beginning at birth) mice. (C and D) Wild-type animals raised under GF conditions. (E and F) CONV-R _Myd88_-/- knockout animals. (A, C, and E) Untreated mice. (B, D, and F) Mice treated for 7 d with 2.5% DSS in their drinking water. (Bars, 150 μm.) (A Inset_–_F Inset) High-power views of sections of crypts stained with H+E (asterisk in A labels a crypt, and the dashed box identifies its associated surface cuff epithelium); PAS/AB to identify mucous-secreting goblet cells (arrows in A); and goat anti-BrdUrd, Alexa Fluor 594-labeled donkey anti-goat Ig (red), and bis-benzimide (blue nuclear stain) to identify cells in S phase (mice were injected with BrdUrd 1 h before being killed). (Bars, 25 μm.) (B Inset and D Inset) Transmission EMs show poorly differentiated ColEPs (B) and terminally differentiated goblet cells with characteristic apical mucin-containing theca cells (D). (G) Quantification of epithelial proliferation and apoptosis. Mean values ± SEM are plotted for each group. An asterisk indicates a value that is significantly different from its untreated control (P < 0.01; Student's t test). (H) Quantification of lymphoctytes (B220; CD3ε), neutrophils (Gr-1), and macrophages (F4/80).
Fig. 2.
Myd88-dependent ColEP response to wounding requires macrophages. (A) Quantification of epithelial proliferation (Left) and apoptosis (Right) in the descending colons of CONV-R B6 wild-type mice transplanted with bone marrow from either wild-type or Myd88-/- donors. Mice were analyzed 12 weeks after bone marrow transplantation, with or without a 7-d exposure to DSS. (B) Quantification of epithelial proliferation (Left) and apoptosis (Right) in mice that lack specific immune cells, including lymphocytes, (_Rag1_-/-), neutrophils (ablated by injection of anti-Gr-1 mAbs), and macrophages (Csf1op/op). An asterisk indicates a statistically significant difference between DSS-treated and untreated mice (P < 0.01; Student's t test).
Fig. 3.
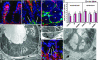
Activated macrophages are required for ColEP response to ulcerated injury. (A and B) Sections of descending colons stained with Alexa Fluor 488-labeled rat anti-F4/80 (green), rabbit anti-laminin, Alexa Fluor 594-labeled donkey anti-rabbit Ig (red), and bis-benzimide (blue). Results are from untreated (A) and DSS-treated CONV-R wild-type mice (B). (Bars, 25 μm.) (B) Arrows show macrophages with cellular extensions around the crypt base in areas with diminished laminin staining. (C) Crosssection through the crypt bases of the descending colon from a CONV-R wild-type, DSS-treated mouse stained with rat anti-CD31 (platelet-endothelial cell adhesion molecule) and Alexa Fluor 350-labeled donkey anti-rat Ig (blue), followed by Alexa Fluor 488-labeled rat anti-F4/80 (green) and Cy3-labeled mouse anti-smooth muscle actin (red). Asterisks identify crypt lumens. (Bar, 25 μm.) (D) Quantification of smooth muscle actin- and CD31-positive cells in untreated and DSS-treated CONV-R and GF wild-type and CONV-R _Myd88_-/- mice. (E) Transmission EM of a crypt base and its surrounding mesenchyme from a CONV-R DSS-treated wild-type mouse. Mesenchymal cells with long cellular processes that appose the crypt are evident (arrowheads). (Bar, 5 μm.) (F)A higher magnification of the cell body of a mesenchymal cell with long processes. The cell has morphologic features consistent with activated macrophages but not blood vessels (lumen-forming) or myofibroblasts (containing cytoplasmic actin bundles). (Bar, 5 μm.) (G) Multilabel immunohistochemistry of a DSS-treated GF wild-type mouse by using Abs to F4/80 (green) and laminin (red). Arrows outline the basal surface of crypt base epithelial cells. Unlike CONV-R DSS-treated mice, recruited macrophages are not juxtaposed against laminin-deficient areas. (Bar, 25 μm.) (H) EM of crypt base and surrounding mesenchyme from a GF DSS-treated mouse. Mesenchymal cells with randomly oriented short cellular processes are evident (arrow). (Bar, 5 μm.)
References
- Chang, W. W. & Leblond, C. P. (1971) Am. J. Anat. 131, 73-99. - PubMed
- Chang, W. W. & Nadler, N. J. (1975) Am. J. Anat. 144, 39-56. - PubMed
- Spradling, A., Drummond-Barbosa, D. & Kai, T. (2001) Nature 414, 12-18. - PubMed
- Fuchs, E., Tumbar, T. & Guasch, G. (2004) Cell 116, 769-778. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK 06220/DK/NIDDK NIH HHS/United States
- U01 DK063483/DK/NIDDK NIH HHS/United States
- DK 63483/DK/NIDDK NIH HHS/United States
- DK 02954/DK/NIDDK NIH HHS/United States
- K08 DK002954/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous