Development of allergic airway disease in mice following antibiotic therapy and fungal microbiota increase: role of host genetics, antigen, and interleukin-13 - PubMed (original) (raw)
Development of allergic airway disease in mice following antibiotic therapy and fungal microbiota increase: role of host genetics, antigen, and interleukin-13
Mairi C Noverr et al. Infect Immun. 2005 Jan.
Abstract
Lending support to the hygiene hypothesis, epidemiological studies have demonstrated that allergic disease correlates with widespread use of antibiotics and alterations in fecal microbiota ("microflora"). Antibiotics also lead to overgrowth of the yeast Candida albicans, which can secrete potent prostaglandin-like immune response modulators, from the microbiota. We have recently developed a mouse model of antibiotic-induced gastrointestinal microbiota disruption that is characterized by stable increases in levels of gastrointestinal enteric bacteria and Candida. Using this model, we have previously demonstrated that microbiota disruption can drive the development of a CD4 T-cell-mediated airway allergic response to mold spore challenge in immunocompetent C57BL/6 mice without previous systemic antigen priming. The studies presented here address important questions concerning the universality of the model. To investigate the role of host genetics, we tested BALB/c mice. As with C57BL/6 mice, microbiota disruption promoted the development of an allergic response in the lungs of BALB/c mice upon subsequent challenge with mold spores. In addition, this allergic response required interleukin-13 (IL-13) (the response was absent in IL-13(-/-) mice). To investigate the role of antigen, we subjected mice with disrupted microbiota to intranasal challenge with ovalbumin (OVA). In the absence of systemic priming, only mice with altered microbiota developed airway allergic responses to OVA. The studies presented here demonstrate that the effects of microbiota disruption are largely independent of host genetics and the nature of the antigen and that IL-13 is required for the airway allergic response that follows microbiota disruption.
Figures
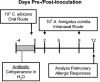
FIG. 1.
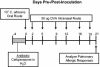
Experimental timeline for conidium challenge model of pulmonary hypersensitivity. BALB/C or IL-13−/− mice were given oral cefoperazone (0.5 mg/ml) for 5 days (day −4 through day 0). At day 0, C. albicans (107 CFU) was administered orally, and mice were challenged (days 2 and 9) by intranasal exposure to A. fumigatus (107 conidia/mouse). Mice were analyzed at day 12 post-C. albicans inoculation.
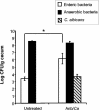
FIG. 2.
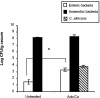
Effect of antibiotic treatment on microbiota populations. At day 12 post-C. albicans inoculation, murine ceca were harvested, homogenized in sterile water, and plated on the following media for enumeration of cecal bacterial populations: VRBA for enteric bacteria, TSA II blood agar for total anaerobic bacteria, and SDA for yeast. Groups are as follows: untreated (conidium challenged, no antibiotic, no C. albicans inoculation) and Anb/Ca (conidium challenged, antibiotic treated, C. albicans oral inoculation). n = 6 to 7 mice per time point pooled from two separate experiments.
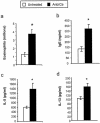
FIG. 3.
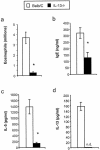
Effects of microbiota perturbation on pulmonary eosinophil recruitment, Th2 cytokine production by lung leukocytes, and serum IgE production in conidium-challenged mice. Both groups of mice were challenged with conidia. Mice were treated as diagrammed Fig. 1. Leukocytes were isolated from lungs by enzymatic digestion and mechanical dispersion. (a) Eosinophils were phenotyped by Wright-Giemsa staining of cytospun samples. Results are expressed as the mean number of leukocytes per mouse ± standard error of the mean. (b) Serum IgE concentrations were measured by ELISA. (c and d) For Th2 cytokine measurements, leukocytes were isolated from whole lungs and cultured for 24 h without additional stimulation. Supernatants were collected and assayed for IL-5 (c) and IL-13 (d) by ELISA. Results are expressed as the mean ± standard error of the mean. n = 7 to 8 mice pooled from two separate experiments. Groups are as follows: untreated (conidium challenged, no antibiotic, no C. albicans inoculation) and Anb/Ca (conidium challenged, antibiotic treated, C. albicans oral inoculation). *, P < 0.05 as determined by Student's t test.
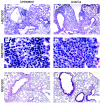
FIG. 4.
Histological analysis of lungs of mice exposed to conidia. Mice were treated as outlined in Fig. 1. At day 12 post-C. albicans inoculation, lungs were harvested, fixed, sectioned, and stained with hematoxylin and eosin (H&E), which differentially stains leukocytes (a-d). Lung sections were also stained with PAS-hematoxylin, which stains mucus pink (e, f). Groups are as follows: untreated (conidium challenged, no antibiotic, no C. albicans inoculation) and Anb/Ca (conidium challenged, antibiotic treated, C. albicans oral inoculation).
FIG. 5.
Effects of microbiota perturbation on pulmonary eosinophil recruitment, Th2 cytokine production by lung leukocytes, and serum IgE production in conidium-challenged IL-13−/− mice. Mice were treated as outlined in Fig. 1. Leukocytes were isolated from lungs by enzymatic digestion and mechanical dispersion. (a) Eosinophils were phenotyped by Wright-Giemsa staining of cytospun samples. Results are expressed as the mean number of leukocytes per mouse ± standard error of the mean. (b) Serum IgE concentrations were measured by ELISA. (c and d) For Th2 cytokine measurements, leukocytes were isolated from whole lungs and cultured for 24 h without additional stimulation. Supernatants were collected and assayed for IL-5 (c) and IL-13 (d) by ELISA. Results are expressed as means ± standard errors of the means. n = 7 to 8 mice pooled from two separate experiments. Both BALB/c and IL-13−/− mice were challenged with A. fumigatus, treated with an antibiotic, and orally inoculated with C. albicans. n.d., not detected. *, P < 0.05 as determined by Student's t test.
FIG. 6.
Histological analysis of lungs of IL-13−/− mice exposed to A. fumigatus. Mice were treated as outlined in Fig. 1. At day 12 post-C. albicans inoculation, lungs were harvested, fixed, sectioned, and stained with hematoxylin and eosin (H&E), which differentially stains leukocytes (a-c). Lung sections were also stained with PAS-methyl green, which stains mucus pink (d, e). Both BALB/c and IL-13−/− mice were challenged with A. fumigatus, treated with an antibiotic, and orally inoculated with C. albicans.
FIG. 7.
Experimental timeline for OVA challenge model of pulmonary hypersensitivity. BALB/c mice were given oral cefoperazone (0.5 mg/ml) for 5 days (day −4 through day 0). At day 0, C. albicans (107 CFU) was administered orally. Mice were challenged (days 2, 5, 9, 12, 16, and 19) with intranasal ovalbumin (50 μg/mouse). Mice were analyzed at day 21 post-C. albicans inoculation.
FIG. 8.
Effect of antibiotic treatment on cecal microbiota populations. At day 21, ceca were harvested and plated on the following media to enumerate cecal bacterial populations: VRBA for enteric bacteria, TSA II blood agar for total anaerobic bacteria, and SDA for yeast. n = 7 mice per time point pooled from two separate experiments. Groups are as follows: untreated (OVA challenged, no antibiotic, no C. albicans inoculation) and Anb/Ca (OVA challenged, antibiotic treated, C. albicans oral inoculation). *, P < 0.05 as determined by Student's t test.
FIG. 9.
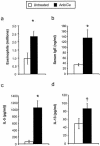
Effects of microbiota perturbation on pulmonary eosinophil recruitment, Th2 cytokine production by lung leukocytes, and serum IgE production in OVA-challenged mice. Both groups of mice were challenged with OVA. Mice were treated as diagrammed in Fig. 7. Leukocytes were isolated from lungs by enzymatic digestion and mechanical dispersion. (a) Eosinophils were phenotyped by Wright-Giemsa staining of cytospun samples. Results are expressed as the mean number of leukocytes per mouse ± standard error of the mean. (b) Serum IgE concentrations were measured by ELISA. (c and d) For Th2 cytokine measurements, leukocytes were isolated from whole lungs and cultured for 24 h without additional stimulation. Supernatants were collected and assayed for IL-5 (c) and IL-13 (d) by ELISA. Results are means ± standard errors of the means. n = 7 mice pooled from two separate experiments. Groups are as follows: untreated (OVA challenged, no antibiotic, no C. albicans inoculation) and Anb/Ca (OVA challenged, antibiotic treated, C. albicans oral inoculation). *, P < 0.05 as determined by Student's t test; †, P < 0.05 as determined by the Mann-Whitney test.
FIG. 10.
Histological analysis of lungs of OVA-challenged mice. Mice were treated to disrupt the microbiota and were challenged intranasally with OVA as outlined in the experimental design (Fig. 7). At day 21 post-C. albicans inoculation, lungs were harvested, fixed, sectioned, and stained with hematoxylin and eosin (H&E), which differentially stains leukocytes (a-c). Lung sections were also stained with PAS-methyl green, which stains mucus pink (d, e). Groups are as follows: untreated (OVA challenged, no antibiotic, no C. albicans inoculation) and Anb/Ca (OVA challenged, antibiotic treated, C. albicans oral inoculation).
Similar articles
- Role of antibiotics and fungal microbiota in driving pulmonary allergic responses.
Noverr MC, Noggle RM, Toews GB, Huffnagle GB. Noverr MC, et al. Infect Immun. 2004 Sep;72(9):4996-5003. doi: 10.1128/IAI.72.9.4996-5003.2004. Infect Immun. 2004. PMID: 15321991 Free PMC article. - [Allergic airway response associated with the intestinal microflora disruption induced by antibiotic therapy].
Liu CH, Yang XQ, Liu CH, He Y, Wang LJ. Liu CH, et al. Zhonghua Er Ke Za Zhi. 2007 Jun;45(6):450-4. Zhonghua Er Ke Za Zhi. 2007. PMID: 17880794 Chinese. - [Pulmonary allergic responses were drived by atomization with ovalbumin in BALB/c mice with intestinal microflora disruption].
Sun DQ, Yang XQ, Liu W, Li X. Sun DQ, et al. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2010 Mar;26(3):281-4. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2010. PMID: 20491174 Chinese. - Expansion of commensal fungus Wallemia mellicola in the gastrointestinal mycobiota enhances the severity of allergic airway disease in mice.
Skalski JH, Limon JJ, Sharma P, Gargus MD, Nguyen C, Tang J, Coelho AL, Hogaboam CM, Crother TR, Underhill DM. Skalski JH, et al. PLoS Pathog. 2018 Sep 20;14(9):e1007260. doi: 10.1371/journal.ppat.1007260. eCollection 2018 Sep. PLoS Pathog. 2018. PMID: 30235351 Free PMC article. - The Role of Early Life Microbiota Composition in the Development of Allergic Diseases.
Tuniyazi M, Li S, Hu X, Fu Y, Zhang N. Tuniyazi M, et al. Microorganisms. 2022 Jun 9;10(6):1190. doi: 10.3390/microorganisms10061190. Microorganisms. 2022. PMID: 35744708 Free PMC article. Review.
Cited by
- The Microbiota in Children and Adolescents with Asthma.
Casali L, Stella GM. Casali L, et al. Children (Basel). 2024 Sep 27;11(10):1175. doi: 10.3390/children11101175. Children (Basel). 2024. PMID: 39457140 Free PMC article. Review. - The role and mechanism of gut-lung axis mediated bidirectional communication in the occurrence and development of chronic obstructive pulmonary disease.
Song X, Dou X, Chang J, Zeng X, Xu Q, Xu C. Song X, et al. Gut Microbes. 2024 Jan-Dec;16(1):2414805. doi: 10.1080/19490976.2024.2414805. Epub 2024 Oct 24. Gut Microbes. 2024. PMID: 39446051 Free PMC article. Review. - Profiling inflammatory outcomes of Candida albicans colonization and food allergy induction in the murine glandular stomach.
Zeise KD, Falkowski NR, Stark KG, Brown CA, Huffnagle GB. Zeise KD, et al. mBio. 2024 Nov 13;15(11):e0211324. doi: 10.1128/mbio.02113-24. Epub 2024 Sep 30. mBio. 2024. PMID: 39347572 Free PMC article. - Antibiotic use during influenza infection augments lung eosinophils that impair immunity against secondary bacterial pneumonia.
Sanches Santos Rizzo Zuttion M, Parimon T, Bora SA, Yao C, Lagree K, Gao CA, Wunderink RG, Kitsios GD, Morris A, Zhang Y, McVerry BJ, Modes ME, Marchevsky AM, Stripp BR, Soto CM, Wang Y, Merene K, Cho S, Victor BL, Vujkovic-Cvijin I, Gupta S, Cassel SL, Sutterwala FS, Devkota S, Underhill DM, Chen P. Sanches Santos Rizzo Zuttion M, et al. J Clin Invest. 2024 Sep 10;134(21):e180986. doi: 10.1172/JCI180986. J Clin Invest. 2024. PMID: 39255040 Free PMC article. - The gut-lung axis: the impact of the gut mycobiome on pulmonary diseases and infections.
Sey EA, Warris A. Sey EA, et al. Oxf Open Immunol. 2024 Jul 24;5(1):iqae008. doi: 10.1093/oxfimm/iqae008. eCollection 2024. Oxf Open Immunol. 2024. PMID: 39193472 Free PMC article. Review.
References
- Akbari, O., R. H. DeKruyff, and D. T. Umetsu. 2001. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat. Immunol. 2:725-731. - PubMed
- Andrew, D. K., R. R. Schellenberg, J. C. Hogg, C. J. Hanna, and P. D. Pare. 1984. Physiological and immunological effects of chronic antigen exposure in immunized guinea pigs. Int. Arch. Allergy Appl. Immunol. 75:208-213. - PubMed
- Calderone, R. A. (ed.) 2001. Candida and candidiasis. ASM Press, Washington, D.C.
- Campbell, E. M., I. F. Charo, S. L. Kunkel, R. M. Strieter, L. Boring, J. Gosling, and N. W. Lukacs. 1999. Monocyte chemoattractant protein-1 mediates cockroach allergen-induced bronchial hyperreactivity in normal but not CCR2−/− mice: the role of mast cells. J. Immunol. 163:2160-2167. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 HL007749/HL/NHLBI NIH HHS/United States
- 2T32HL007749-11/HL/NHLBI NIH HHS/United States
- R01 AI059201/AI/NIAID NIH HHS/United States
- R01-HL65912/HL/NHLBI NIH HHS/United States
- R01-AI059201/AI/NIAID NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- R01 HL065912/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials