The periplasmic alpha-carbonic anhydrase activity of Helicobacter pylori is essential for acid acclimation - PubMed (original) (raw)
The periplasmic alpha-carbonic anhydrase activity of Helicobacter pylori is essential for acid acclimation
Elizabeth A Marcus et al. J Bacteriol. 2005 Jan.
Abstract
The role of the periplasmic alpha-carbonic anhydrase (alpha-CA) (HP1186) in acid acclimation of Helicobacter pylori was investigated. Urease and urea influx through UreI have been shown to be essential for gastric colonization and for acid survival in vitro. Intrabacterial urease generation of NH3 has a major role in regulation of periplasmic pH and inner membrane potential under acidic conditions, allowing adequate bioenergetics for survival and growth. Since alpha-CA catalyzes the conversion of CO2 to HCO3-, the role of CO2 in periplasmic buffering was studied using an alpha-CA deletion mutant and the CA inhibitor acetazolamide. Western analysis confirmed that alpha-CA was bound to the inner membrane. Immunoblots and PCR confirmed the absence of the enzyme and the gene in the alpha-CA knockout. In the mutant or in the presence of acetazolamide, there was an approximately 3 log10 decrease in acid survival. In acid, absence of alpha-CA activity decreased membrane integrity, as observed using membrane-permeant and -impermeant fluorescent DNA dyes. The increase in membrane potential and cytoplasmic buffering following urea addition to wild-type organisms in acid was absent in the alpha-CA knockout mutant and in the presence of acetazolamide, although UreI and urease remained fully functional. At low pH, the elevation of cytoplasmic and periplasmic pH with urea was abolished in the absence of alpha-CA activity. Hence, buffering of the periplasm to a pH consistent with viability depends not only on NH3 efflux from the cytoplasm but also on the conversion of CO2, produced by urease, to HCO3- by the periplasmic alpha-CA.
Figures
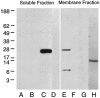
FIG. 1.
Western blot analysis of α-CA. Membrane and soluble proteins were prepared from wild-type H. pylori and the H. pylori α-CA deletion mutant as described in Materials and Methods. Immunoblot analysis failed to detect α-CA in the soluble protein fraction from either the wild-type or mutant bacteria (lanes A and B, respectively). A band with an apparent molecular mass of 28 kDa was detected in the wild-type H. pylori membrane preparation (lane E) but not in the membrane fraction prepared from the α-CA deletion mutant (lane F). UreA was detected in the soluble fraction (lane C) but not in the membrane fraction (lane G). UreI was detected in the membrane fraction (lane H) but not in the soluble fraction (lane D).
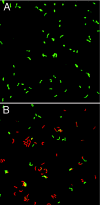
FIG. 2.
Periplasmic CA activity is required for inner membrane integrity in acidic medium. α-CA deletion mutant bacteria were incubated for 30 min with urea in the presence of Syto9 (Live/Dead; Molecular Probes) and propidium iodide (Live/Dead) at pH 7.4 and pH 3.0. (A) No uptake of the positively charged propidium iodide was observed at pH 7.4. (B) At pH 3.0 the deletion mutant showed a large increase in propidium iodide uptake (red fluorescence), indicating loss of membrane in integrity.
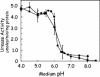
FIG. 3.
α-CA expression is not required for UreI activation of urea entry. Steady-state urease activity as a function of different fixed medium pHs revealed a normal acid activation curve in the α-CAKO mutant (▪). There was about a 20-fold increase in intrabacterial urease activity between pH 7.0 and 5.0, with half-maximal activation at ∼pH 6.0, as previously found for wild-type organisms (•).
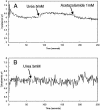
FIG. 4.
α-CA activity is required to increase membrane potential following urea addition in acid. (A) At pHout 3.0, the addition of urea increases membrane potential from −10 to −101 mV in wild-type H. pylori due to alkalization of the periplasm. (B) Urea addition had no effect on the membrane potential of the α-CAKO mutant. (C) The increase in membrane potential of the wild-type H. pylori following urea addition was reversed by the addition of 1 mM acetazolamide.
FIG. 5.
Cytoplasmic pH in the wild type and α-CAKO mutant. Cytoplasmic pH was determined with the pH-sensitive, fluorescent dye BCECF-AM. (A) In acidic medium (pH 5.0), urea addition raises the cytoplasmic pH of wild-type H. pylori. (B) In the absence of α-CA activity, either by gene deletion or by enzyme inhibition, urea fails to increase intrabacterial pH.
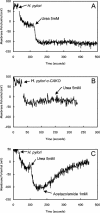
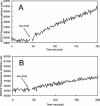
FIG. 6.
Fluorimetric measurement of the rate of change of medium pH following the addition of urea to wild-type or mutant organisms. Medium pH was measured at pH 5.0 in weak buffer by the change of fluorescence of BCECF free acid. (A) In wild-type H. pylori, a transient increase in fluorescence followed by a steady increase was observed. In the HP1186 knockout mutant, the transient increase was not observed and the rate of medium alkalinization was slowed due to decreased cytoplasmic pH observed at pH 5.0 in the absence of periplasmic CA activity.
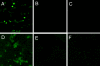
FIG. 7.
Visualization of periplasmic alkalinization in H. pylori. Using confocal microscopy in the presence of BCECF free acid, it is possible to visualize changes in periplasmic pH since BCECF penetrates the outer, but not the inner, membrane. BCECF fluorescence increases with increasing pH. Ninety seconds after the addition of 5 mM urea the pH of the periplasm of wild-type H. pylori increased (A) but not that of the α-CAKO mutant (B) or with inhibition of α-CA by acetazolamide (C). After 5 min, the pH of the medium increased in all conditions, indicating retention of urease activity in the absence of α-CA activity (D, E, and F).
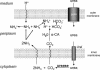
FIG. 8.
A model for the role of urease and α-CA in maintenance of periplasmic pH in medium acidity. Urea enters from the medium into the periplasm presumably by diffusion across outer membrane porins. Under acidic conditions, urea moves into the cytoplasm due to acid gating of urea entry through the UreI channel. CO2 and 2NH3 are then produced by urease activity in the cytoplasm. These gases diffuse rapidly into the periplasm. In the periplasm, CO2 is hydrated rapidly to H2CO3 by α-CA and dissociates to H+ and HCO3−, with the proton being consumed by 1NH3 to form NH4+. The additional NH3 produced by urease can neutralize some of the acid entering the periplasm. This model accounts for the ability of intrabacterial urease activity to neutralize entering acid and at the same time maintain a pH of ∼6.1 in the periplasm.
Similar articles
- Cytoplasmic histidine kinase (HP0244)-regulated assembly of urease with UreI, a channel for urea and its metabolites, CO2, NH3, and NH4(+), is necessary for acid survival of Helicobacter pylori.
Scott DR, Marcus EA, Wen Y, Singh S, Feng J, Sachs G. Scott DR, et al. J Bacteriol. 2010 Jan;192(1):94-103. doi: 10.1128/JB.00848-09. J Bacteriol. 2010. PMID: 19854893 Free PMC article. - Local pH elevation mediated by the intrabacterial urease of Helicobacter pylori cocultured with gastric cells.
Athmann C, Zeng N, Kang T, Marcus EA, Scott DR, Rektorschek M, Buhmann A, Melchers K, Sachs G. Athmann C, et al. J Clin Invest. 2000 Aug;106(3):339-47. doi: 10.1172/JCI9351. J Clin Invest. 2000. PMID: 10930437 Free PMC article. - Roles of alpha and beta carbonic anhydrases of Helicobacter pylori in the urease-dependent response to acidity and in colonization of the murine gastric mucosa.
Bury-Moné S, Mendz GL, Ball GE, Thibonnier M, Stingl K, Ecobichon C, Avé P, Huerre M, Labigne A, Thiberge JM, De Reuse H. Bury-Moné S, et al. Infect Immun. 2008 Feb;76(2):497-509. doi: 10.1128/IAI.00993-07. Epub 2007 Nov 19. Infect Immun. 2008. PMID: 18025096 Free PMC article. - The gastric biology of Helicobacter pylori.
Sachs G, Weeks DL, Melchers K, Scott DR. Sachs G, et al. Annu Rev Physiol. 2003;65:349-69. doi: 10.1146/annurev.physiol.65.092101.142156. Epub 2002 May 1. Annu Rev Physiol. 2003. PMID: 12471160 Review. - Acid acclimation by Helicobacter pylori.
Sachs G, Weeks DL, Wen Y, Marcus EA, Scott DR, Melchers K. Sachs G, et al. Physiology (Bethesda). 2005 Dec;20:429-38. doi: 10.1152/physiol.00032.2005. Physiology (Bethesda). 2005. PMID: 16287992 Review.
Cited by
- Identification of metal dithiocarbamates as a novel class of antileishmanial agents.
Pal DS, Mondal DK, Datta R. Pal DS, et al. Antimicrob Agents Chemother. 2015 Apr;59(4):2144-52. doi: 10.1128/AAC.05146-14. Epub 2015 Jan 26. Antimicrob Agents Chemother. 2015. PMID: 25624329 Free PMC article. - Role of the Helicobacter pylori sensor kinase ArsS in protein trafficking and acid acclimation.
Marcus EA, Sachs G, Wen Y, Feng J, Scott DR. Marcus EA, et al. J Bacteriol. 2012 Oct;194(20):5545-51. doi: 10.1128/JB.01263-12. Epub 2012 Aug 3. J Bacteriol. 2012. PMID: 22865848 Free PMC article. - Phosphorylation-dependent and Phosphorylation-independent Regulation of Helicobacter pylori Acid Acclimation by the ArsRS Two-component System.
Marcus EA, Sachs G, Wen Y, Scott DR. Marcus EA, et al. Helicobacter. 2016 Feb;21(1):69-81. doi: 10.1111/hel.12235. Epub 2015 May 22. Helicobacter. 2016. PMID: 25997502 Free PMC article. - Structural and functional aspects of the Helicobacter pylori secretome.
Zanotti G, Cendron L. Zanotti G, et al. World J Gastroenterol. 2014 Feb 14;20(6):1402-23. doi: 10.3748/wjg.v20.i6.1402. World J Gastroenterol. 2014. PMID: 24587618 Free PMC article. Review. - Functional characterization of Actinobacillus pleuropneumoniae urea transport protein, ApUT.
Godara G, Smith C, Bosse J, Zeidel M, Mathai J. Godara G, et al. Am J Physiol Regul Integr Comp Physiol. 2009 Apr;296(4):R1268-73. doi: 10.1152/ajpregu.90726.2008. Epub 2009 Jan 14. Am J Physiol Regul Integr Comp Physiol. 2009. PMID: 19144751 Free PMC article.
References
- Akada, J. K., M. Shirai, H. Takeuchi, M. Tsuda, and T. Nakazawa. 2000. Identification of the urease operon in Helicobacter pylori and its control by mRNA decay in response to pH. Mol Microbiol. 36:1071-1084. - PubMed
- Baumgartner, H. K., and M. H. Montrose. 2004. Regulated alkali secretion acts in tandem with unstirred layers to regulate mouse gastric surface pH. Gastroenterology 126:774-783. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK053642/DK/NIDDK NIH HHS/United States
- R01 DK-41301/DK/NIDDK NIH HHS/United States
- R01 DK-46917/DK/NIDDK NIH HHS/United States
- R01 DK-53642/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources