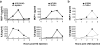Toll-like receptor 9 mediates innate immune activation by the malaria pigment hemozoin - PubMed (original) (raw)
Comparative Study
. 2005 Jan 3;201(1):19-25.
doi: 10.1084/jem.20041836.
Ken J Ishii, Taro Kawai, Hiroaki Hemmi, Shintaro Sato, Satoshi Uematsu, Masahiro Yamamoto, Osamu Takeuchi, Sawako Itagaki, Nirbhay Kumar, Toshihiro Horii, Shizuo Akira
Affiliations
- PMID: 15630134
- PMCID: PMC2212757
- DOI: 10.1084/jem.20041836
Comparative Study
Toll-like receptor 9 mediates innate immune activation by the malaria pigment hemozoin
Cevayir Coban et al. J Exp Med. 2005.
Abstract
Malaria parasites within red blood cells digest host hemoglobin into a hydrophobic heme polymer, known as hemozoin (HZ), which is subsequently released into the blood stream and then captured by and concentrated in the reticulo-endothelial system. Accumulating evidence suggests that HZ is immunologically active, but the molecular mechanism(s) through which HZ modulates the innate immune system has not been elucidated. This work demonstrates that HZ purified from Plasmodium falciparum is a novel non-DNA ligand for Toll-like receptor (TLR)9. HZ activated innate immune responses in vivo and in vitro, resulting in the production of cytokines, chemokines, and up-regulation of costimulatory molecules. Such responses were severely impaired in TLR9-/- and myeloid differentiation factor 88 (MyD88)-/-, but not in TLR2, TLR4, TLR7, or Toll/interleukin 1 receptor domain-containing adaptor-inducing interferon beta-/- mice. Synthetic HZ, which is free of the other contaminants, also activated innate immune responses in vivo in a TLR9-dependent manner. Chloroquine (CQ), an antimalarial drug, abrogated HZ-induced cytokine production. These data suggest that TLR9-mediated, MyD88-dependent, and CQ-sensitive innate immune activation by HZ may play an important role in malaria parasite-host interactions.
Figures
Figure 1.
Purified HZ from P. falciparum activates proinflammatory responses through MyD88-dependent pathway. (a) FL-DCs from wild-type (WT) mice were stimulated with 30 and 100 μM of purified HZ for 24 h. ELISA was performed to measure TNFα, IL-12p40, MCP-1, or IL-6 production in the supernatants. As a control, 3 μM CpG DNA (D35) was used. (b) FL-DCs and (c) spleen cells from wild-type (solid bars) and MyD88−/− mice (open bars) were stimulated with 30 μM HZ and 3 μM CpG DNA (D35) or 100 ng/ml LPS for 24 h. Production of TNFα, IL-12p40, MCP-1, and IL-6 was measured by ELISA in the culture supernatant. (d) The myeloid DC (CD11c+ B220−) and plasmacytoid DCs (CD11c+ B220+) were analyzed for CD40 and CD86 expression by flow cytometry. The shaded area represents nonstimulated cells, and the solid line represents stimulated cells with HZ. Results represent the mean ± SD of duplicate cultures and are representative of at least five independent experiments. n.d., not detected.
Figure 2.
HZ-induced DC activation is TRIF independent. FL-DCs from wild-type (WT) and TRIF−/− mice were incubated with 30 μM HZ and 100 ng/ml LPS for 24 h, after which supernatants were assayed by ELISA for TNFα or IL-12p40 production. Results are the mean ± SEM of four independent experiments (n = 4 mice). P > 0.05, HZ (WT) versus HZ (TRIF−/−). n.d., not detected.
Figure 3.
TLR9 mediates HZ-induced innate immune activation. (a) The myeloid (CD11c+ B220−) and plasmacytoid DCs (CD11c+ B220+) from wild-type (WT) and TLR2−/−, TLR4−/−, TLR7−/−, or TLR9−/− mice were analyzed for CD40 and CD86 expression by flow cytometry. The shaded area represents nonstimulated cells, and the solid line represents stimulated cells with HZ. Wild-type and TLR9−/− cells were incubated with the indicated stimuli and analyzed for (b) TNFα, IL-12p40, MCP-1, or IL-6 production by spleen cells and (c) IFNα production by FL-DCs by ELISA. Results are representative of at least five independent experiments. n.d., not detected.
Figure 4.
Serum cytokine productions by HZ and synthetic HZ (β-hematin) in vivo are dependent on MyD88 and TLR9. MyD88−/−, TLR9−/−, or wild-type (WT) mice were injected i.p. with either 1,500 μg of (a) purified P. falciparum HZ or (b) synthetic HZ (sHZ). Serum levels of MCP-1 and IL-6 were measured by ELISA at the indicated time points.
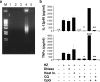
Figure 5.
HZ is free of DNA, and HZ-induced proinflammatory cytokine production is DNase and heat resistant, but CQ sensitive. (a) HZ was run on an agarose gel and ethidium bromide stained to detect possible DNA contamination. M, marker; lane 1, HZ solution (1 mM, 5 μl); lane 2, heat-inactivated HZ (1 mM, 5 μl); lane 3, DNase-treated HZ (1 mM, 5 μl); lane 4, P. falciparum crude extract (5 μl from packed 100 ml of culture containing 4.5% parasitemia); lane 5, DNase-treated crude extract (5 μl). (b) FL-DCs from C57/B6 mice was incubated with either 30 μM HZ or with DNase treatment, heat inactivation, and/or 10 μM CQ for 24 h. Supernatants were then collected and measured for TNFα or IL-12p40 by ELISA. As a control, 3 μM CpG ODN (D35) was used. The value of TNFα by D35 is shown. Results are representative of one of three independent experiments performed in duplicate (mean ± SD). *, P < 0.05, HZ versus HZ plus CQ; **, P < 0.05, CpG ODN versus CpG ODN plus CQ. n.d., not detected.
Similar articles
- The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors.
Horng T, Barton GM, Flavell RA, Medzhitov R. Horng T, et al. Nature. 2002 Nov 21;420(6913):329-33. doi: 10.1038/nature01180. Nature. 2002. PMID: 12447442 - Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4.
Yamamoto M, Sato S, Hemmi H, Sanjo H, Uematsu S, Kaisho T, Hoshino K, Takeuchi O, Kobayashi M, Fujita T, Takeda K, Akira S. Yamamoto M, et al. Nature. 2002 Nov 21;420(6913):324-9. doi: 10.1038/nature01182. Nature. 2002. PMID: 12447441 - Interleukin-1 receptor-associated kinase-1 plays an essential role for Toll-like receptor (TLR)7- and TLR9-mediated interferon-{alpha} induction.
Uematsu S, Sato S, Yamamoto M, Hirotani T, Kato H, Takeshita F, Matsuda M, Coban C, Ishii KJ, Kawai T, Takeuchi O, Akira S. Uematsu S, et al. J Exp Med. 2005 Mar 21;201(6):915-23. doi: 10.1084/jem.20042372. Epub 2005 Mar 14. J Exp Med. 2005. PMID: 15767370 Free PMC article. - The immunobiology of the TLR9 subfamily.
Wagner H. Wagner H. Trends Immunol. 2004 Jul;25(7):381-6. doi: 10.1016/j.it.2004.04.011. Trends Immunol. 2004. PMID: 15207506 Review. No abstract available. - Regulation of innate immune responses by Toll-like receptors.
Takeda K, Akira S. Takeda K, et al. Jpn J Infect Dis. 2001 Dec;54(6):209-19. Jpn J Infect Dis. 2001. PMID: 11862002 Review.
Cited by
- Polymorphisms in the Fc gamma receptor IIIA and Toll-like receptor 9 are associated with protection against severe malarial anemia and changes in circulating gamma interferon levels.
Munde EO, Okeyo WA, Anyona SB, Raballah E, Konah S, Okumu W, Ogonda L, Vulule J, Ouma C. Munde EO, et al. Infect Immun. 2012 Dec;80(12):4435-43. doi: 10.1128/IAI.00945-12. Epub 2012 Oct 8. Infect Immun. 2012. PMID: 23045477 Free PMC article. - Methemoglobin-induced signaling and chemokine responses in human alveolar epithelial cells.
Mumby S, Ramakrishnan L, Evans TW, Griffiths MJ, Quinlan GJ. Mumby S, et al. Am J Physiol Lung Cell Mol Physiol. 2014 Jan 1;306(1):L88-100. doi: 10.1152/ajplung.00066.2013. Epub 2013 Oct 18. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 24142518 Free PMC article. - Malarial hemozoin: from target to tool.
Coronado LM, Nadovich CT, Spadafora C. Coronado LM, et al. Biochim Biophys Acta. 2014 Jun;1840(6):2032-41. doi: 10.1016/j.bbagen.2014.02.009. Epub 2014 Feb 17. Biochim Biophys Acta. 2014. PMID: 24556123 Free PMC article. Review. - Hemozoin inhibition and control of clinical malaria.
Ihekwereme CP, Esimone CO, Nwanegbo EC. Ihekwereme CP, et al. Adv Pharmacol Sci. 2014;2014:984150. doi: 10.1155/2014/984150. Epub 2014 Feb 9. Adv Pharmacol Sci. 2014. PMID: 24669217 Free PMC article. Review. - Innate immunity to Toxoplasma gondii infection.
Yarovinsky F. Yarovinsky F. Nat Rev Immunol. 2014 Feb;14(2):109-21. doi: 10.1038/nri3598. Nat Rev Immunol. 2014. PMID: 24457485 Review.
References
- Miller, L.H., and S.L. Hoffman. 1998. Research toward vaccines against malaria. Nat. Med. 4:520–524. - PubMed
- Stevenson, M.M., and E.M. Riley. 2004. Innate immunity to malaria. Nat. Rev. Immunol. 4:169–180. - PubMed
- Kwiatkowski, D., C.A. Bate, I.G. Scragg, P. Beattie, I. Udalova, and J.C. Knight. 1997. The malarial fever response–pathogenesis, polymorphism and prospects for intervention. Ann. Trop. Med. Parasitol. 91:533–542. - PubMed
- Janeway, C.A., Jr., and R. Medzhitov. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197–216. - PubMed
- Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499–511. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases