Regeneration of the pancreatic beta cell - PubMed (original) (raw)
Review
Regeneration of the pancreatic beta cell
Massimo Trucco. J Clin Invest. 2005 Jan.
Abstract
Type 1 diabetes is the result of an autoimmune attack against the insulin-producing beta cells of the endocrine pancreas. Current treatment for patients with type 1 diabetes typically involves a rigorous and invasive regimen of testing blood glucose levels many times a day along with subcutaneous injections of recombinant DNA-derived insulin. Islet transplantation, even with its substantially improved outcome in recent years, is still not indicated for pediatric patients. However, in light of the fact that some regenerative capabilities of the endocrine pancreas have been documented and recent research has shown that human ES cell lines can be derived in vitro, this review discusses whether it is practical or even possible to combine these lines of research to more effectively treat young diabetic patients.
Figures
Figure 1
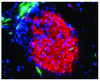
Cross section of the pancreas. The pancreas houses 2 distinctly different tissues. Its bulk comprises exocrine tissue, which is made up of acinar cells that secrete pancreatic enzymes delivered to the intestine to facilitate the digestion of food. Scattered throughout the exocrine tissue are many thousands of clusters of endocrine cells known as islets of Langerhans. Within the islet, α cells produce glucagon; β cells, insulin; δ cells, somatostatin; and γ cells, pancreatic polypeptide – all of which are delivered to the blood.
Figure 2
Regeneration of the β cell in diabetic NOD mice. (A) In NOD mice, the infiltration of autoreactive T cells into the islets of Langerhans (resulting in insulitis) begins at around 4 weeks of age. At 20 to 23 weeks, approximately 85% of female mice are diabetic, i.e., their glycemia is greater than 300 mg/dl. Magnification, ×200. (B) When it is successfully transplanted with bone marrow from a non–diabetes-prone donor and hematopoietic chimerism is established, the NOD mouse no longer show signs of autoimmune activity. However, while there is no more evidence of insulitis in the endogenous pancreas, there is also no sign of insulin production (no red staining). Magnification, ×400. (C) Insulin-positive cells in the islets can be seen to be dividing (yellow arrows); i.e., they are insulin (blue) and BrdU (red) positive. Magnification, ×400. (D) Three to 4 months after bone marrow transplantation, new insulin-positive cells (shown in red) are present throughout the endogenous pancreas. Magnification, ×200. Thus, when the islets transplanted under the kidney capsule in order to maintain euglycemia while regeneration takes place are removed by nephrectomy, the mice remain nondiabetic. Figure reproduced with permission from Stem Cells (33).
Figure 3
Using a GFP-transgenic mouse as donor, it is possible to observe how the majority of the transplanted bone marrow cells do not directly participate in the regeneration of the endogenous pancreas. As shown here, there are no double-positive (orange) cells in the newly formed islets. The donor cells (green) appear to be located close to possibly existing juxta-ductal precursor cells, which may be activated by bone marrow cell–secreted factors. Insulin-positive cells are red. Magnification, ×400. See also refs. and .
Figure 4
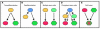
Classification of stem cells based on their developmental potential according to Wagers and Weissman (44). Totipotent, able to give rise to all embryonic and extraembryonic cell types; pluripotent, able to give rise to all cell types of the embryo proper; multipotent, able to give rise to a subset of cell lineages; oligopotent, able to give rise to a restricted subset of cell lineages; unipotent, able to contribute only one mature cell type.
Figure 5
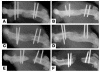
Radiographic evaluation at 4 and 12 weeks after surgery. The critical-sized (i.e., non–spontaneously reparable) defect in the femora treated with bone marrow–derived stromal cells transfected with retro-BMP4 exhibited a notable bridging callus (i.e., the white mass between the 2 extremes of the fracture interval) at both 4 (A) and 12 (B) weeks after surgery. The defect in the femora treated with MDCs transfected with BMP4 had also developed a bridging callus at 4 (C) and 12 (D) weeks. No bone formation was radiographically evident in the control – i.e., femora treated with MDCs transfected with the LacZ gene – at both 4 (E) and 12 (F) weeks after surgery. Figure reproduced with permission from Langenbecks Archives of Surgery (55).
Figure 6
Schematic diagram depicting potential and known mechanisms of adult stem cell plasticity (A–E). Orange or green ovals, tissue-specific stem cells; blue ovals, pluripotent stem cells; red ovals and green hexagons, differentiated cells of tissue-specific (orange or green) lineages. Figure reproduced with permission from Cell (44).
Figure 7
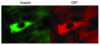
Duodenal sections from transgenic mice harboring the GIP/Ins transgene. The K cells of the gut examined by immunofluorescence microscopy showed both human insulin production (green) and expression of the glucose-dependent insulinotropic polypeptide (red). Figure reproduced with permission from Science (63).
Similar articles
- Islet replacement vs. regeneration: hope or hype?
Ramiya V, Schatz D. Ramiya V, et al. Pediatr Diabetes. 2004;5 Suppl 2:45-56. doi: 10.1111/j.1399-543X.2004.00079.x. Pediatr Diabetes. 2004. PMID: 15601374 Review. - Islet regeneration during the reversal of autoimmune diabetes in NOD mice.
Kodama S, Kühtreiber W, Fujimura S, Dale EA, Faustman DL. Kodama S, et al. Science. 2003 Nov 14;302(5648):1223-7. doi: 10.1126/science.1088949. Science. 2003. PMID: 14615542 - Stem cell-based approaches to solving the problem of tissue supply for islet transplantation in type 1 diabetes.
Street CN, Sipione S, Helms L, Binette T, Rajotte RV, Bleackley RC, Korbutt GS. Street CN, et al. Int J Biochem Cell Biol. 2004 Apr;36(4):667-83. doi: 10.1016/j.biocel.2003.09.005. Int J Biochem Cell Biol. 2004. PMID: 15010331 Review. - β-Cell differentiation and regeneration in type 1 diabetes.
Ding L, Gysemans C, Mathieu C. Ding L, et al. Diabetes Obes Metab. 2013 Sep;15 Suppl 3:98-104. doi: 10.1111/dom.12164. Diabetes Obes Metab. 2013. PMID: 24003926 Review. - Beta-cell replacement and regeneration: Strategies of cell-based therapy for type 1 diabetes mellitus.
Limbert C, Päth G, Jakob F, Seufert J. Limbert C, et al. Diabetes Res Clin Pract. 2008 Mar;79(3):389-99. doi: 10.1016/j.diabres.2007.06.016. Epub 2007 Sep 12. Diabetes Res Clin Pract. 2008. PMID: 17854943 Review.
Cited by
- A matching algorithm for the distribution of human pancreatic islets.
Qian D, Kaddis J, Niland JC. Qian D, et al. Comput Stat Data Anal. 2007 Aug;51(12):5494-5506. doi: 10.1016/j.csda.2007.02.030. Comput Stat Data Anal. 2007. PMID: 22199413 Free PMC article. - Pancreatic islet cell therapy for type I diabetes: understanding the effects of glucose stimulation on islets in order to produce better islets for transplantation.
Ren J, Jin P, Wang E, Liu E, Harlan DM, Li X, Stroncek DF. Ren J, et al. J Transl Med. 2007 Jan 3;5:1. doi: 10.1186/1479-5876-5-1. J Transl Med. 2007. PMID: 17201925 Free PMC article. Review. - Therapeutics for type-2 diabetes mellitus: a glance at the recent inclusions and novel agents under development for use in clinical practice.
Shah N, Abdalla MA, Deshmukh H, Sathyapalan T. Shah N, et al. Ther Adv Endocrinol Metab. 2021 Sep 23;12:20420188211042145. doi: 10.1177/20420188211042145. eCollection 2021. Ther Adv Endocrinol Metab. 2021. PMID: 34589201 Free PMC article. Review. - The current status of islet transplantation and its perspectives.
Kobayashi N. Kobayashi N. Rev Diabet Stud. 2008 Fall;5(3):136-43. doi: 10.1900/RDS.2008.5.136. Epub 2008 Nov 10. Rev Diabet Stud. 2008. PMID: 19099085 Free PMC article. - Compromised central tolerance of ICA69 induces multiple organ autoimmunity.
Fan Y, Gualtierotti G, Tajima A, Grupillo M, Coppola A, He J, Bertera S, Owens G, Pietropaolo M, Rudert WA, Trucco M. Fan Y, et al. J Autoimmun. 2014 Sep;53:10-25. doi: 10.1016/j.jaut.2014.07.001. Epub 2014 Aug 1. J Autoimmun. 2014. PMID: 25088457 Free PMC article.
References
- Wells JM. Genes expressed in the developing endocrine pancreas and their importance for stem cell and diabetes research. Diabetes Metab. Res. Rev. 2003;19:191–201. - PubMed
- Jensen J. Gene regulatory factors in pancreatic development. Dev. Dyn. 2004;229:176–200. - PubMed
- Bach JF. Insulin-dependent diabetes mellitus as an autoimmune disease. Endocr. Rev. 1994;15:516–542. - PubMed
- Lacy PE. Pancreatic transplantation as a means of insulin delivery. Diabetes Care. 1982;5(Suppl. 1):93–97. - PubMed
- Shapiro AM, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 2000;343:230–238. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical