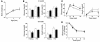PKClambda regulates glucose-induced insulin secretion through modulation of gene expression in pancreatic beta cells - PubMed (original) (raw)
. 2005 Jan;115(1):138-45.
doi: 10.1172/JCI22232.
Yoshiaki Kido, Tohru Uchida, Tomokazu Matsuda, Kazuhisa Suzuki, Hiroshi Inoue, Michihiro Matsumoto, Wataru Ogawa, Sakan Maeda, Hiroaki Fujihara, Yoichi Ueta, Yasuo Uchiyama, Kazunori Akimoto, Shigeo Ohno, Tetsuo Noda, Masato Kasuga
Affiliations
- PMID: 15630453
- PMCID: PMC539193
- DOI: 10.1172/JCI22232
PKClambda regulates glucose-induced insulin secretion through modulation of gene expression in pancreatic beta cells
Naoko Hashimoto et al. J Clin Invest. 2005 Jan.
Abstract
Altered regulation of insulin secretion by glucose is characteristic of individuals with type 2 diabetes mellitus, although the mechanisms that underlie this change remain unclear. We have now generated mice that lack the lambda isoform of PKC in pancreatic beta cells (betaPKClambda(-/-) mice) and show that these animals manifest impaired glucose tolerance and hypoinsulinemia. Furthermore, insulin secretion in response to high concentrations of glucose was impaired, whereas the basal rate of insulin release was increased, in islets isolated from betaPKClambda(-/-) mice. Neither the beta cell mass nor the islet insulin content of betaPKClambda(-/-) mice differed from that of control mice, however. The abundance of mRNAs for Glut2 and HNF3beta was reduced in islets of betaPKClambda(-/-) mice, and the expression of genes regulated by HNF3beta was also affected (that of Sur1 and Kir6.2 genes was reduced, whereas that of hexokinase 1 and hexokinase 2 genes was increased). Normalization of HNF3beta expression by infection of islets from betaPKClambda(-/-) mice with an adenoviral vector significantly reversed the defect in glucose-stimulated insulin secretion. These results indicate that PKClambda plays a prominent role in regulation of glucose-induced insulin secretion by modulating the expression of genes important for beta cell function.
Figures
Figure 1
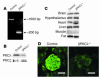
Generation of β cell–specific PKCλ knockout mice. (A) PCR analysis of genomic DNA isolated from islets of control (PKC_λ_flox/flox) and βPKCλ–/– mice. The primers were targeted to regions external to the loxP sites of PKC_λ_flox/flox mice. (B and C) Immunoblot analysis of PKCλ in the islets (B) or the brain, hypothalamus, heart, liver, skeletal muscle, and fat (C) of control and βPKCλ–/– mice. Tissue homogenates were subjected to immunoprecipitation and subsequent immunoblot analysis with antibodies against PKCλ. The expression of PKCλ in islets was similarly analyzed. (D) Immunostaining of islets in pancreatic sections of control and βPKCλ–/– mice with antibodies against PKCλ and FITC-conjugated secondary antibodies. Scale bars: 50 μm.
Figure 2
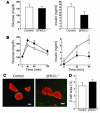
Effect of β cell–specific ablation of PKCλ on glucose metabolism. (A) Growth curves of control (open circles) and βPKCλ–/– (filled circles) mice. Mice were weighed at 2, 4, and 6 months. (B) Blood glucose and plasma insulin concentrations of 6-month-old control (white bars) and βPKCλ–/– (black bars) mice in the fasting or fed state. (C) Intraperitoneal glucose tolerance tests performed in control and βPKCλ–/– mice that had fasted overnight. (D) Blood glucose concentrations during insulin tolerance testing in control and βPKCλ–/– mice. Data are means ± SE of values from 25 (A), 28 (B), 10 (C), or 6 (D) animals of each genotype. *P < 0.05 (ANOVA) versus the corresponding value for control mice.
Figure 3
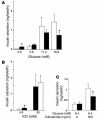
Impairment of glucose-stimulated insulin secretion in isolated islets of βPKCλ–/– mice. Insulin release in response to the indicated concentrations of glucose (A), KCl (B), or tolbutamide (C) was measured with islets isolated from control (white bars) or βPKCλ–/– (black bars) mice. Data are means ± SE of values from 6 animals of each genotype. *P < 0.05 (ANOVA) versus the corresponding value for control mice.
Figure 4
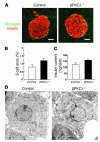
Lack of effect of β cell–specific ablation of PKCλ on islet morphology and insulin content. (A) Immunostaining of pancreatic sections from 6-month-old control and βPKCλ–/– mice with antibodies against insulin (red) and glucagon (green). Scale bars: 50 μm. (B) Quantitation of β cell area as a percentage of total pancreatic area in control and βPKCλ–/– mice. Data are means ± SE of values from 4 mice of each genotype. (C) Insulin content of isolated islets. Data are means ± SE of values from 4 mice of each genotype. (D) Electron microscopy of β cells of control and βPKCλ–/– mice. Magnification, ×10,000.
Figure 5
Effects of a high-fat diet on β cell phenotype in βPKCλ–/– mice. (A) Blood glucose and plasma insulin concentrations in the fed state of control and βPKCλ–/– mice on a high-fat diet. Data are means ± SE of values from 12 animals of each genotype. (B) Intraperitoneal glucose tolerance tests in control (open circles) and βPKCλ–/– (filled circles) mice after 15 weeks on the high-fat diet. Data are means ± SE of values from 6 animals of each genotype. *P < 0.05 (ANOVA) versus the corresponding value for control mice. (C) Immunostaining of pancreatic sections from control and βPKCλ–/– mice after 15 weeks on the high-fat diet with antibodies against insulin (red) and glucagon (green). Scale bars: 100 μm. (D) Quantitation of β cell area as a percentage of total pancreatic area in control and βPKCλ–/– mice after 15 weeks on the high-fat diet. Data are means ± SE of values from 3 mice of each genotype.
Figure 6
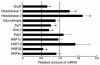
Altered gene expression in the islets of βPKCλ–/– mice. The abundance of mRNAs for the indicated proteins was determined by real-time RT-PCR analysis of total RNA isolated from islets of control and βPKCλ–/– mice. The amounts of the mRNAs in βPKCλ–/– mice are expressed relative to those in control animals. Data are means ± SE of triplicates for pooled total RNA samples from 6 mice of each genotype and are representative of a total of 3 similar experiments. *P < 0.05 (ANOVA) versus the corresponding value (1.0) for control mice.
Figure 7
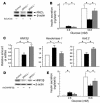
Effects of adenovirus-mediated restoration of PKCλ or HNF3β expression on insulin secretion in islets of βPKCλ–/– mice. (A–C) Islets isolated from control mice or βPKCλ–/– mice were infected with an adenovirus encoding either β-galactosidase (AxCALacZ) or wild-type PKCλ (AxCAλwt). The islets were then either subjected to immunoblot analysis with antibodies against PKCλ or β actin (A); assayed for insulin secretion in the presence of 2.8 or 16.8 mM glucose (white bars, control islets plus AxCALacZ; black bars, βPKCλ–/– islets plus AxCALacZ; gray bars, βPKCλ–/– islets plus AxCAλwt) (B); or subjected to real-time RT-PCR analysis of mRNAs for HNF3β, hexokinase 1, or Kir6.2 (C). (D and E) Islets isolated from control or βPKCλ–/– mice were infected with either AxCALacZ or an adenovirus encoding wild-type HNF3β (AxCAHNF3β). The islets were then either subjected to immunoblot analysis with antibodies against HNF3β or β actin (D) or assayed for insulin secretion in the presence of 2.8 or 16.8 mM glucose (E). Data are means ± SE of values from 6 mice (B and E) or of triplicates for pooled total RNA samples from 5 mice (C). *P < 0.05 (ANOVA) for the indicated comparisons.
Comment in
- CaV2.3 channel and PKClambda: new players in insulin secretion.
Yang SN, Berggren PO. Yang SN, et al. J Clin Invest. 2005 Jan;115(1):16-20. doi: 10.1172/JCI23970. J Clin Invest. 2005. PMID: 15630435 Free PMC article.
Similar articles
- Foxa2 regulates multiple pathways of insulin secretion.
Lantz KA, Vatamaniuk MZ, Brestelli JE, Friedman JR, Matschinsky FM, Kaestner KH. Lantz KA, et al. J Clin Invest. 2004 Aug;114(4):512-20. doi: 10.1172/JCI21149. J Clin Invest. 2004. PMID: 15314688 Free PMC article. - Hepatocyte nuclear factor 4alpha regulates the expression of pancreatic beta -cell genes implicated in glucose metabolism and nutrient-induced insulin secretion.
Wang H, Maechler P, Antinozzi PA, Hagenfeldt KA, Wollheim CB. Wang H, et al. J Biol Chem. 2000 Nov 17;275(46):35953-9. doi: 10.1074/jbc.M006612200. J Biol Chem. 2000. PMID: 10967120 - Desensitization of the insulin-secreting beta cell.
Grodsky GM, Bolaffi JL. Grodsky GM, et al. J Cell Biochem. 1992 Jan;48(1):3-11. doi: 10.1002/jcb.240480103. J Cell Biochem. 1992. PMID: 1316359 Review. - Potential role of peroxisome proliferator-activated receptor-alpha in the modulation of glucose-stimulated insulin secretion.
Sugden MC, Holness MJ. Sugden MC, et al. Diabetes. 2004 Feb;53 Suppl 1:S71-81. doi: 10.2337/diabetes.53.2007.s71. Diabetes. 2004. PMID: 14749269 Review.
Cited by
- Ablation of TSC2 enhances insulin secretion by increasing the number of mitochondria through activation of mTORC1.
Koyanagi M, Asahara S, Matsuda T, Hashimoto N, Shigeyama Y, Shibutani Y, Kanno A, Fuchita M, Mikami T, Hosooka T, Inoue H, Matsumoto M, Koike M, Uchiyama Y, Noda T, Seino S, Kasuga M, Kido Y. Koyanagi M, et al. PLoS One. 2011;6(8):e23238. doi: 10.1371/journal.pone.0023238. Epub 2011 Aug 19. PLoS One. 2011. PMID: 21886784 Free PMC article. - ABCC8 and ABCC9: ABC transporters that regulate K+ channels.
Bryan J, Muñoz A, Zhang X, Düfer M, Drews G, Krippeit-Drews P, Aguilar-Bryan L. Bryan J, et al. Pflugers Arch. 2007 Feb;453(5):703-18. doi: 10.1007/s00424-006-0116-z. Epub 2006 Aug 8. Pflugers Arch. 2007. PMID: 16897043 Review. - Ablation of C/EBPbeta alleviates ER stress and pancreatic beta cell failure through the GRP78 chaperone in mice.
Matsuda T, Kido Y, Asahara S, Kaisho T, Tanaka T, Hashimoto N, Shigeyama Y, Takeda A, Inoue T, Shibutani Y, Koyanagi M, Hosooka T, Matsumoto M, Inoue H, Uchida T, Koike M, Uchiyama Y, Akira S, Kasuga M. Matsuda T, et al. J Clin Invest. 2010 Jan;120(1):115-26. doi: 10.1172/JCI39721. Epub 2009 Dec 1. J Clin Invest. 2010. PMID: 19955657 Free PMC article. - Muscle-specific knockout of PKC-lambda impairs glucose transport and induces metabolic and diabetic syndromes.
Farese RV, Sajan MP, Yang H, Li P, Mastorides S, Gower WR Jr, Nimal S, Choi CS, Kim S, Shulman GI, Kahn CR, Braun U, Leitges M. Farese RV, et al. J Clin Invest. 2007 Aug;117(8):2289-301. doi: 10.1172/JCI31408. J Clin Invest. 2007. PMID: 17641777 Free PMC article. - Overexpression of KLF15 transcription factor in adipocytes of mice results in down-regulation of SCD1 protein expression in adipocytes and consequent enhancement of glucose-induced insulin secretion.
Nagare T, Sakaue H, Matsumoto M, Cao Y, Inagaki K, Sakai M, Takashima Y, Nakamura K, Mori T, Okada Y, Matsuki Y, Watanabe E, Ikeda K, Taguchi R, Kamimura N, Ohta S, Hiramatsu R, Kasuga M. Nagare T, et al. J Biol Chem. 2011 Oct 28;286(43):37458-69. doi: 10.1074/jbc.M111.242651. Epub 2011 Aug 23. J Biol Chem. 2011. PMID: 21862590 Free PMC article.
References
- Rhodes CJ. IGF-I and GH post-receptor signaling mechanisms for pancreatic beta-cell replication. J. Mol. Endocrinol. 2000;24:303–311. - PubMed
- Withers DJ, et al. Irs-2 coordinates Igf-1 receptor-mediated beta-cell development and peripheral insulin signalling. Nat. Genet. 1999;23:32–40. - PubMed
- Leibiger IB, Leibiger B, Moede T, Berggren PO. Exocytosis of insulin promotes insulin gene transcription via the insulin receptor/PI-3 kinase/p70 S6 kinase and CaM kinase pathways. Mol. Cell. 1998;1:933–938. - PubMed
- Leibiger B, et al. Selective insulin signaling through A and B insulin receptors regulates transcription of insulin and glucokinase genes in pancreatic beta cells. Mol. Cell. 2001;7:559–570. - PubMed
- Persaud SJ, Harris TE, Burns CJ, Jones PM. Tyrosine kinases play a permissive role in glucose-induced insulin secretion from adult rat islets. J. Mol. Endocrinol. 1999;22:19–28. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases