Synergistic increases in intracellular Ca2+, and the release of MCP-1, RANTES, and IL-6 by astrocytes treated with opiates and HIV-1 Tat - PubMed (original) (raw)
Synergistic increases in intracellular Ca2+, and the release of MCP-1, RANTES, and IL-6 by astrocytes treated with opiates and HIV-1 Tat
Nazira El-Hage et al. Glia. 2005.
Abstract
Recent evidence suggests that injection drug users who abuse heroin are at increased risk of CNS complications from human immunodeficiency virus (HIV) infection. Opiate drugs may intrinsically alter the pathogenesis of HIV by directly modulating immune function and by directly modifying the CNS response to HIV. Despite this, the mechanisms by which opiates increase the neuropathogenesis of HIV are uncertain. In the present study, we describe the effect of morphine and the HIV-1 protein toxin Tat(1-72) on astroglial function in cultures derived from ICR mice. Astroglia maintain the blood-brain barrier and influence inflammatory signaling in the CNS. Astrocytes can express mu-opioid receptors, and are likely targets for abused opiates, which preferentially activate mu-opioid receptors. While Tat alone disrupts astrocyte function, when combined with morphine, Tat causes synergistic increases in [Ca(2+)](i). Moreover, astrocyte cultures treated with morphine and Tat showed exaggerated increases in chemokine release, including monocyte chemoattractant protein-1 (MCP-1) and regulated on activation, normal T cell expressed and secreted (RANTES), as well as interleukin-6 (IL-6). Morphine-Tat interactions were prevented by the mu-opioid receptor antagonist beta-funaltrexamine, or by immunoneutralizing Tat(1-72) or substituting a nontoxic, deletion mutant (Tat(Delta31-61)). Our findings suggest that opiates may increase the vulnerability of the CNS to viral entry (via recruitment of monocytes/macrophages) and ensuing HIV encephalitis by synergistically increasing MCP-1 and RANTES release by astrocytes. The results further suggest that astrocytes are key intermediaries in opiate-HIV interactions and disruptions in astroglial function and inflammatory signaling may contribute to an accelerated neuropathogenesis in HIV-infected individuals who abuse opiates.
(c) 2004 Wiley-Liss, Inc.
Figures
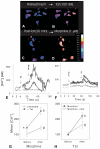
Figure 1
Effect of morphine (Morph) and/or Tat1-72 on intracellular calcium [Ca2+]i in flat, polyhedral (type 1) astroglia as assessed by ratiometric analysis using fura-2 (A-H). Morphine (1 μM) and/or Tat (100 nM) alone significantly increased [Ca2+]i in individual type 1 astrocytes (A-B,E-G), compared to mean baseline levels (96.5 ± 6.4 nM). Alternatively, pretreatment with Tat1-72 (100 nM) exacerbated the subsequent increases in astroglial [Ca2+]i to morphine (1 μM) (E), and visa versa (F). The responses differed dramatically among individual astrocytes; some cells responded synergistically (thick line), while others did not (thin dashed-line). In type 1 astroglial populations, morphine (1 μM) (*P < 0.05) or Tat1-72 (100 nM) (P < 0.05) alone significantly increased mean [Ca2+]i; levels; however, in combination mean [Ca2+]i levels increased synergistically (b_P_ < 0.027; ANOVA, two-way interaction) (G). The interactive increases in [Ca2+]i caused by morphine and Tat1-72 (b_P_ < 0.0024, pretreatment vs. morphine + Tat post-treatment) were significantly attenuated by naloxone pretreatment (3 μM for 1 h) (#P < 0.05) (h). Data in G & H are the mean ± SEM from 7 experiments; in each experiment ~35 astrocytes were arbitrarily sampled (~250 astrocytes total).
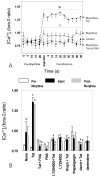
Figure 2
Inhibition of morphine (Morph) and/and Tat1-72-induced increases in intracellular calcium [Ca2+]i by thapsigargin, dantrolene, and the PI3-kinase inhibitor, LY294002. Cells were incubated with serum free medium or stimulated with 500 nM morphine ± 100 nM Tat1-72 in the presence or absence of 1.5 μM β-FNA. Cells were pre-incubated with the specific inhibitor of phosphatidylinositol 3-kinase (LY294002) (5 μM), the Ca2+-ionophore A23187 (1 μM), thapsigargin (5 μM) (thaps), or dantrolene (10 μM) (dantr) for 20 min before stimulation of the cells with morphine and or Tat1-72. Bars represent the mean ± SEM of four independent experiments (*P < 0.05 versus vehicle-treated control cultures; #P < 0.05 versus morphine + Tat1-72-treated cultures; bP < 0.05 versus both vehicle-treated control and morphine + Tat1-72-treated cultures).
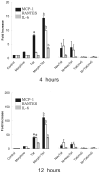
Figure 3
Effects of morphine (500 nM) and/or Tat1-72 (100 nM) on cytokine release by astrocytes at 4 h (A) and 12 h (B) following continuous exposure. Controls for Tat1-72 included 100 nM deletion (TatΔ31-61) mutant Tat [Tat(mut)] and 100 nM immunoneutralized Tat1-72 (Neutral Tat) administered in the presence or absence of morphine. Supernatants were collected and analyzed for cytokines using TranSignal ™ RayBiotech mouse cytokine antibody arrays; the rectangles in the upper left and lower right portions of the arrays indicate positive and negative controls; while the centrally positioned rectangles indicate several cytokines that showed specific responses to morphine and/or Tat (IL-6, MCP-1, and RANTES).
Figure 4
Effects of morphine (500 nM) and/or Tat1-72 (100 nM) on cytokine release by astrocytes at 4 h (A) and 12 h (B) following continuous exposure. Neither TatΔ31-61 [Tat(mut)] (100 nM) nor immunoneutralized Tat1-72 (NeuTat) alone or in combination with morphine (100 nM) affected the release of cytokines.
Figure 5
Time-dependent effects of morphine and Tat1-72 on the expression of MCP-1 and RANTES mRNA levels in astrocytes assayed by RT-PCR. Cells were cultured with or without morphine (500 nM) (Morph), Tat1-72 (100 nM), or morphine plus Tat1-72 for 30 min, 4 or 12 h. RNA was extracted, reverse transcribed, and amplified by PCR with the primers as indicated in material and methods. PCR products were separated on a 1.5 % agarose gel. β-actin was used as a housekeeping gene and its expression remained unchanged. The expression levels of different chemokine mRNAs were normalized to β-actin mRNA, and fold-induction was calculated relative to cells incubated in culture medium (vehicle) alone as described in the methods. The μ-opioid receptor antagonist β-FNA reversed morphine-induced exacerbation of Tat1-72 effects on MCP-1 and RANTES gene expression in cultured astrocytes suggesting the effects of morphine were mediated through μ-opioid receptors; while β-FNA had no effect on Tat-induced changes in the absence of morphine. Cells were incubated ± morphine (Morph or Mor), Tat1-72, and/or β-funaltrexamine (FNA) for 4 or 12 h. Bars represent the mean ± SEM from at least three independent experiments (*P < 0.05 versus vehicle-treated controls; #P < 0.05 versus morphine + Tat1-72-treated cultures; b_P_ < 0.05 versus both vehicle-treated control and Tat1-72-treated cultures; Kruskal-Wallis test).
Figure 6
Time-dependent effects of morphine and Tat1-72 on the expression of TNF-α and IL-6 mRNA levels in astrocytes assayed by RT-PCR at 30 min (A,B) or 4 h or 12 h (C,D). RNA was analyzed by RT-PCR, normalized to β-actin mRNA levels, and fold induction was calculated in comparison to the cells in cultured medium alone as described in Fig. 5. β-actin expression was unaffected by any of the treatments. Bars represent the mean ± SEM from at least three independent experiments (*P < 0.05 versus vehicle-treated controls; #P < 0.05 versus morphine + Tat1-72-treated cultures; bP < 0.05 versus both vehicle-treated control and Tat1-72-treated cultures; Kruskal-Wallis test).
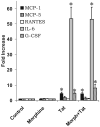
Figure 7
Effects of morphine (500 nM) and/or Tat1-72 (100 nM) on cytokine release by microglia at 18 h following continuous exposure. Tat alone caused significant increases in MCP-1 (*P > 0.05), and in IL-6 and G-CSF levels (*_P_>0.05 versus vehicle-treated controls; Kruskal-Wallis test), while the addition of morphine had no added effect beyond that seen with Tat alone. Unlike findings at 18 h, microglia showed minimal release of cytokines in response to Tat and/or morphine at 12 h following continuous exposure (see text). Therefore, the response of microglia to morphine and/or Tat differed markedly from the response of astroglia.
Similar articles
- HIV-1 Tat and opiate-induced changes in astrocytes promote chemotaxis of microglia through the expression of MCP-1 and alternative chemokines.
El-Hage N, Wu G, Wang J, Ambati J, Knapp PE, Reed JL, Bruce-Keller AJ, Hauser KF. El-Hage N, et al. Glia. 2006 Jan 15;53(2):132-46. doi: 10.1002/glia.20262. Glia. 2006. PMID: 16206161 Free PMC article. - CCL5/RANTES gene deletion attenuates opioid-induced increases in glial CCL2/MCP-1 immunoreactivity and activation in HIV-1 Tat-exposed mice.
El-Hage N, Bruce-Keller AJ, Knapp PE, Hauser KF. El-Hage N, et al. J Neuroimmune Pharmacol. 2008 Dec;3(4):275-85. doi: 10.1007/s11481-008-9127-1. Epub 2008 Sep 25. J Neuroimmune Pharmacol. 2008. PMID: 18815890 Free PMC article. - Preferential vulnerability of astroglia and glial precursors to combined opioid and HIV-1 Tat exposure in vitro.
Khurdayan VK, Buch S, El-Hage N, Lutz SE, Goebel SM, Singh IN, Knapp PE, Turchan-Cholewo J, Nath A, Hauser KF. Khurdayan VK, et al. Eur J Neurosci. 2004 Jun;19(12):3171-82. doi: 10.1111/j.0953-816X.2004.03461.x. Eur J Neurosci. 2004. PMID: 15217373 Free PMC article. - Opiate drug use and the pathophysiology of neuroAIDS.
Hauser KF, Fitting S, Dever SM, Podhaizer EM, Knapp PE. Hauser KF, et al. Curr HIV Res. 2012 Jul;10(5):435-52. doi: 10.2174/157016212802138779. Curr HIV Res. 2012. PMID: 22591368 Free PMC article. Review. - Role of mu-opioids as cofactors in human immunodeficiency virus type 1 disease progression and neuropathogenesis.
Banerjee A, Strazza M, Wigdahl B, Pirrone V, Meucci O, Nonnemacher MR. Banerjee A, et al. J Neurovirol. 2011 Aug;17(4):291-302. doi: 10.1007/s13365-011-0037-2. Epub 2011 Jul 7. J Neurovirol. 2011. PMID: 21735315 Free PMC article. Review.
Cited by
- Evidence for selection at HIV host susceptibility genes in a West Central African human population.
Zhao K, Ishida Y, Oleksyk TK, Winkler CA, Roca AL. Zhao K, et al. BMC Evol Biol. 2012 Dec 6;12:237. doi: 10.1186/1471-2148-12-237. BMC Evol Biol. 2012. PMID: 23217182 Free PMC article. - Morphine efficacy is altered in conditional HIV-1 Tat transgenic mice.
Fitting S, Scoggins KL, Xu R, Dever SM, Knapp PE, Dewey WL, Hauser KF. Fitting S, et al. Eur J Pharmacol. 2012 Aug 15;689(1-3):96-103. doi: 10.1016/j.ejphar.2012.05.029. Epub 2012 May 30. Eur J Pharmacol. 2012. PMID: 22659585 Free PMC article. - GSK3β-activation is a point of convergence for HIV-1 and opiate-mediated interactive neurotoxicity.
Masvekar RR, El-Hage N, Hauser KF, Knapp PE. Masvekar RR, et al. Mol Cell Neurosci. 2015 Mar;65:11-20. doi: 10.1016/j.mcn.2015.01.001. Epub 2015 Jan 20. Mol Cell Neurosci. 2015. PMID: 25616162 Free PMC article. - Gene expression profiles of HIV-1-infected glia and brain: toward better understanding of the role of astrocytes in HIV-1-associated neurocognitive disorders.
Borjabad A, Brooks AI, Volsky DJ. Borjabad A, et al. J Neuroimmune Pharmacol. 2010 Mar;5(1):44-62. doi: 10.1007/s11481-009-9167-1. Epub 2009 Aug 21. J Neuroimmune Pharmacol. 2010. PMID: 19697136 Free PMC article. Review. - beta-Chemokine production by neural and glial progenitor cells is enhanced by HIV-1 Tat: effects on microglial migration.
Hahn YK, Vo P, Fitting S, Block ML, Hauser KF, Knapp PE. Hahn YK, et al. J Neurochem. 2010 Jul;114(1):97-109. doi: 10.1111/j.1471-4159.2010.06744.x. Epub 2010 Apr 9. J Neurochem. 2010. PMID: 20403075 Free PMC article.
References
- Arora PK. Morphine-induced immune modulation: does it predispose to HIV infection? NIDA Res Monogr. 1990;96:150–165. - PubMed
- Bagasra O, Lavi E, Bobroski L, Khalili K, Pestaner JP, Tawadros R, Pomerantz RJ. Cellular reservoirs of HIV-1 in the central nervous system of infected individuals: identification by the combination of in situ polymerase chain reaction and immunohistochemistry. AIDS. 1996;10:573–585. - PubMed
- Bansal AK, Mactutus CF, Nath A, Maragos W, Hauser KF, Booze RM. Neurotoxicity of HIV-1 proteins gp120 and tat in the rat striatum. Brain Res. 2000;879:42–49. - PubMed
- Barker SA, Lujan D, Wilson BS. Multiple roles for PI 3-kinase in the regulation of PLCgamma activity and Ca2+ mobilization in antigen-stimulated mast cells. J Leukoc Biol. 1999;65:321–329. - PubMed
- Bell JE, Brettle RP, Chiswick A, Simmonds P. HIV encephalitis, proviral load and dementia in drug users and homosexuals with AIDS. Effect of neocortical involvement. Brain. 1998;121:2043–2052. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous