Simultaneous imaging of different focal planes in fluorescence microscopy for the study of cellular dynamics in three dimensions - PubMed (original) (raw)
Simultaneous imaging of different focal planes in fluorescence microscopy for the study of cellular dynamics in three dimensions
Prashant Prabhat et al. IEEE Trans Nanobioscience. 2004 Dec.
Abstract
The imaging of cellular dynamics in three dimensions using a standard microscope is severely limited due to the fact that only one focal plane can be imaged at a given point in time. Here we present a modification of the classical microscope design with which two or more focal planes can be imaged simultaneously. This is achieved by a modification of the emission pathway of a standard microscope. The efficacy of the design is shown by imaging bead samples and an FcRn-green fluorescent protein expressing tubule that leaves a sorting endosome and subsequently exocytoses at the plasma membrane.
Figures
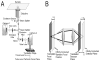
Fig. 1
(A) Layout of microscope configuration. The emission path is split by a beam splitter into two paths. In each of those two paths emission filters are followed by a tube lens that focuses the image onto the detector. One focusing method is provided by the focusing mechanism of the microscope (not shown) by changing the position of the objective with respect to the sample. The focal plane that is imaged with camera 1 is adjusted in this way. The focal plane that is imaged by camera 2 is additionally adjustable through the position of the translation stage on which camera 2 is mounted. This translation stage changes the distance of the detector of camera 2 with respect to the tube lens. In contrast, the detector of camera 1 is located at the focal plane of its tube lens. If the detector of camera 2 is also put at the focal plane of the tube lens both cameras will image the same focal plane in the sample. Changing the position of camera 2 away from the focal plane of the tube lens changes the focal plane in the sample that is imaged by camera 2. Moving camera 2 towards the tube lens results in imaging planes in the sample that are further away from the cover glass. (B) Sketch of emission light path that shows how a detector position different from the typical infinity corrected position results in the imaging of a focal plane that is further from the cover glass than the focal plane corresponding to the standard configuration.
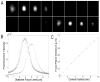
Fig. 2
(A) Montage of images taken of a 100 nm diameter fluorescent bead with the two camera system for the imaging of different focal planes (Fig. 1). Camera 1 was positioned at the focal plane of the tube lens, whereas Camera 2 was positioned 8.8 mm away from the focal position towards the tube lens corresponding to a 1 _μ_m distance between the two focal planes (based on calibration data obtained from Fig. 2C). The top row shows images acquired by Camera 2 whereas the bottom row shows images acquired by Camera 1. The images were taken at different positions of the z-focus of the objective (from left to right the z-focus positions of the objective were: 1.125 _μ_m, 1.375 _μ_m, 1.625 _μ_m, 1.875 _μ_m, 2.125 _μ_m, 2.375 _μ_m, 2.625 _μ_m, 2.875 _μ_m). It is clearly seen that the bead is in focus at different planes for the two cameras, thereby confirming that the acquisition system allows the imaging of different focal planes. (B) Fluorescent intensities of the images acquired as in (A) plotted against objective z-focus levels. Here images are analyzed that were acquired at 25nm increments. The fluorescence intensity plots have different peaks indicating that the focal planes for the two cameras are different. (C) Measurement of the distance between the peaks of the plots in (B) gives a measurement of the difference in the focal planes between Camera 1 and Camera 2. For different positions of Camera 2 analyses of the images were carried out as in (B) that result in estimates of the difference in focal planes between the two cameras. The current plot reveals a linear relationship between the position of Camera 2 (X [mm]) and the difference in focal planes between the two cameras (Y [μ_m]), given by Y = 0.113_X
Fig. 3
Time lapse images acquired with dual plane configuration of endothelial (HMEC-1) cell cotransfected with both FcRn-GFP and FcRn-RFP. One focal plane was set to image (images are labeled ‘L’) the plasma membrane (in TIRFM with a GFP specific filter set); the second focal plane (images are labeled ‘U’) was set to image the sorting endosome level (0.88 _μ_m above in widefield with a RFP specific filter set). The two images in the top left hand corner show a larger part of the cell with the sorting endosome marked by the box in both the upper and lower planes. The frame in image 10 shows the excerpt that is presented for the subsequent images. Images are shown for both focal planes. The number in the bottom right hand corner indicates the time of acquisition (in seconds). The frames in the upper level show how a tubule leaves the sorting endosome (images 1U–6U). This tubule then breaks up (images 9U–10U). One of the two resulting tubules (arrow) then starts to leave the sorting endosome level and appears in the membrane level (image 14 onwards) until it has completely disappeared from the sorting endosome level (image 21U). After arriving on the membrane the tubule partially exocytoses (27L–30L). The fluorescence intensity plots confirm this partial fusion event. Size bars equal 2 _μ_m.
Similar articles
- High-speed confocal fluorescence imaging with a novel line scanning microscope.
Wolleschensky R, Zimmermann B, Kempe M. Wolleschensky R, et al. J Biomed Opt. 2006 Nov-Dec;11(6):064011. doi: 10.1117/1.2402110. J Biomed Opt. 2006. PMID: 17212534 - Toward the clinical application of time-domain fluorescence lifetime imaging.
Munro I, McGinty J, Galletly N, Requejo-Isidro J, Lanigan PM, Elson DS, Dunsby C, Neil MA, Lever MJ, Stamp GW, French PM. Munro I, et al. J Biomed Opt. 2005 Sep-Oct;10(5):051403. doi: 10.1117/1.2102807. J Biomed Opt. 2005. PMID: 16292940 - Improved single particle localization accuracy with dual objective multifocal plane microscopy.
Ram S, Prabhat P, Ward ES, Ober RJ. Ram S, et al. Opt Express. 2009 Apr 13;17(8):6881-98. doi: 10.1364/oe.17.006881. Opt Express. 2009. PMID: 19365515 Free PMC article. - Live cell spinning disk microscopy.
Gräf R, Rietdorf J, Zimmermann T. Gräf R, et al. Adv Biochem Eng Biotechnol. 2005;95:57-75. doi: 10.1007/b102210. Adv Biochem Eng Biotechnol. 2005. PMID: 16080265 Review. - Combined video fluorescence and 3D electron microscopy.
Mironov AA, Polishchuk RS, Beznoussenko GV. Mironov AA, et al. Methods Cell Biol. 2008;88:83-95. doi: 10.1016/S0091-679X(08)00405-6. Methods Cell Biol. 2008. PMID: 18617029 Review.
Cited by
- Depth-resolved cellular microrheology using HiLo microscopy.
Michaelson J, Choi H, So P, Huang H. Michaelson J, et al. Biomed Opt Express. 2012 Jun 1;3(6):1241-55. doi: 10.1364/BOE.3.001241. Epub 2012 May 3. Biomed Opt Express. 2012. PMID: 22741071 Free PMC article. - High-contrast multifocus microscopy with a single camera and z-splitter prism.
Xiao S, Gritton H, Tseng HA, Zemel D, Han X, Mertz J. Xiao S, et al. Optica. 2020 Nov 20;7(11):1477-1486. doi: 10.1364/optica.404678. Epub 2020 Oct 22. Optica. 2020. PMID: 34532564 Free PMC article. - Characteristic rotational behaviors of rod-shaped cargo revealed by automated five-dimensional single particle tracking.
Chen K, Gu Y, Sun W, Bin Dong, Wang G, Fan X, Xia T, Fang N. Chen K, et al. Nat Commun. 2017 Oct 12;8(1):887. doi: 10.1038/s41467-017-01001-9. Nat Commun. 2017. PMID: 29026088 Free PMC article. - Fast, multiplane line-scan confocal microscopy using axially distributed slits.
Tsang JM, Gritton HJ, Das SL, Weber TD, Chen CS, Han X, Mertz J. Tsang JM, et al. Biomed Opt Express. 2021 Feb 9;12(3):1339-1350. doi: 10.1364/BOE.417286. eCollection 2021 Mar 1. Biomed Opt Express. 2021. PMID: 33796357 Free PMC article. - Targeting FcRn for the modulation of antibody dynamics.
Ward ES, Devanaboyina SC, Ober RJ. Ward ES, et al. Mol Immunol. 2015 Oct;67(2 Pt A):131-41. doi: 10.1016/j.molimm.2015.02.007. Epub 2015 Mar 9. Mol Immunol. 2015. PMID: 25766596 Free PMC article. Review.
References
- Inoue S, Spring KR. Video Microscopy: The fundamentals. 2. Plenum Press; 1997.
- Ober RJ, Martinez C, Vacarro C, Ward ES. Visualizing the site and dynamics of IgG salvage by the MHC class I related receptor, FcRn. Journal of Immunology. 2004;172:2021–2029. - PubMed
- Pruckler M, Lawley TJ, Ades EW. Use of a human microvascular endothelial cell line as a model system to evaluate cholestoral uptake. Pathobiology. 1993;61:283–287. - PubMed
- Axelrod D. Surface fluorescence microscopy with evanescent illumination. In: Lacey AJ, editor. Light microscopy in biology. Oxford University Press; 1999. pp. 399–423.
Publication types
MeSH terms
Substances
Grants and funding
- R21 AI 53748/AI/NIAID NIH HHS/United States
- R01 AI050747/AI/NIAID NIH HHS/United States
- R01 AI039167-12/AI/NIAID NIH HHS/United States
- R01 AI039167/AI/NIAID NIH HHS/United States
- R21 AI053748/AI/NIAID NIH HHS/United States
- R01 AI 50747/AI/NIAID NIH HHS/United States
- R01 AI050747-03/AI/NIAID NIH HHS/United States
- R21 AI053748-02/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources