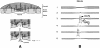Decreased thickness and integrity of the macular elastic layer of Bruch's membrane correspond to the distribution of lesions associated with age-related macular degeneration - PubMed (original) (raw)
Decreased thickness and integrity of the macular elastic layer of Bruch's membrane correspond to the distribution of lesions associated with age-related macular degeneration
N H Victor Chong et al. Am J Pathol. 2005 Jan.
Abstract
Age-related macular degeneration (AMD) is a leading cause of blindness in the elderly. In its severest form, choroidal neovessels breach the macular Bruch's membrane, an extracellular matrix compartment comprised of elastin and collagen laminae, and grow into the retina. We sought to determine whether structural properties of the elastic lamina (EL) correspond to the region of the macula that is predilected toward degeneration in AMD. Morphometric assessment of the macular and extramacular regions of 121 human donor eyes, with and without AMD, revealed a statistically significant difference in both the integrity (P < 0.0001) and thickness (P < 0.0001) of the EL between the macular and extramacular regions in donors of all ages. The EL was three to six times thinner and two to five times less abundant in the macula than in the periphery. The integrity of the macular EL was significantly lower in donors with early-stage AMD (P = 0.028), active choroidal neovascularization (P = 0.020), and disciform scars (P = 0.003), as compared to unaffected, age-matched controls. EL thickness was significantly lower only in individuals with disciform scars (P = 0.008). The largest gaps in macular EL integrity were significantly larger in all categories of AMD (each P < 0.0001), as compared to controls. EL integrity, thickness, and gap length in donors with geographic atrophy did not differ from those of controls. These structural properties of the macular EL correspond spatially to the distribution of macular lesions associated with AMD and may help to explain why the macula is more susceptible to degenerative events that occur in this disease.
Figures
Figure 1
Ocular regions used in this study. A: Color funduscopic photograph of the left eye of a middle-aged individual without any clinical signs of AMD. The macula (dashed circle) is centered on the fovea (arrow) and lies within the major retinal vessels (arrowheads) that emanate from the optic nerve head (ONH). The superior (S), inferior (I), temporal (T), and nasal (N) axes are marked for orientation. B: Diagrammatic representation of the distribution of the 2-mm-diameter retinal-choroidal-scleral punches collected from a 14-year-old donor. The solid black circle depicts the location of the optic nerve head, the curved black lines emanating from the optic disk represent the major retinal vessels, and the blue circle denotes the punch that was centered on the fovea. The remaining circles represent serial 2-mm-diameter punches taken along the superior, inferior, temporal, and nasal axes. C: Illustration showing the macular and extramacular (mid-peripheral) sites (circles) from which 4-mm-diameter punches were collected for EL thickness and integrity measurements in the large set of AMD and unaffected donors. Landmarks are the same as those described in B.
Figure 2
Confocal (A, B) and transmission electron microscopical (C–F) images of the elastic layer of Bruch’s membrane. Images were collected from macular (A, C, E) and extramacular (B, D, F) regions of Bruch’s membrane, as indicated in Figure 1. A and B: Labeling with an antibody directed against tropoelastin in a 78-year-old donor without macular disease. Tissues prepared for confocal microscopy were counterstained with TO-PRO-3 to visualize cellular nuclei (blue); RPE-associated lipofuscin is autofluorescent (red). Note that the elastin immunoreactivity of the elastic layer (green) is far more robust in the extramacular region (B) that it is in the macular region (A). The EL (arrows) in the macular region of an 82-year-old donor, depicted in C and D, is thinner and contains more numerous discontinuities than in the extramacular region. E: A higher magnification image of Bruch’s membrane directly adjacent to the foveal pit; the elastin fibers in this region are very sparse and thin (arrow). When the extramacular EL is viewed en face (F), its porosity (asterisk) is evident. These discontinuities contribute to the integrity and gap length values measured in this study. Original magnifications, ×400 (A, B). Scale bar, 2 μm (C, D).
Figure 3
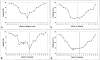
Values for mean integrity (percent; A, B) and thickness (nm; C, D) of the elastic layer derived from the 14-year-old donor described in Figure 1B. Plots A and C depict measurements taken from the inferior ora serrata to the superior ora serrata, passing through the fovea (0), whereas plots B and D show measurements spanning between the nasal and temporal ora serrata, passing through the fovea (0) and optic nerve head (brackets; −5 mm to −7 mm). Note the extremely low integrity of the EL in the fovea (A and B; spanning between −2 mm and +2 mm) and adjacent to the optic nerve head on the nasal side (−8 mm to −10 mm in B). A step increase in integrity occurs in the vicinity of the major retinal vessels (∼−6 mm and +6 mm in A and B). The EL is thinnest in the macula area and increases abruptly in the region of the vascular arcades (∼−6 mm to +6 mm in C and D).
Figure 4
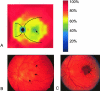
A: A derived, color-coded schematic of the integrity of the elastic layer (relative percent scale shown to right) of Bruch’s membrane based on the data shown in Figure 3, A and B. The black circle represents the optic nerve head and the curved black lines emanating from it are the approximate positions of the major retinal vessels. Note that the integrity is lowest in the foveal region (arrow), central macula, and on the nasal aspect of the optic nerve head. These regions of decreased integrity correspond spatially to the distribution of the majority of macular lesions, such as GA (B; arrows depict the margin of macular atrophy) and CNVMs (C; arrow) that occur in individuals with AMD.
Figure 5
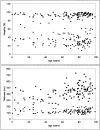
Scatter plots showing values of EL integrity (top; percent) and thickness (bottom; nm) derived from 121 individual donors, aged 1 day to 99 years. Note that the integrity of the EL in the macular region is approximately three times lower, on average, than that of the extramacular regions in all donors. The thickness of the EL in the macular regions is approximately four to six times lower, on average, than that of the extramacular region in all donors.
Figure 6
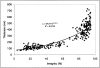
Scatter plot showing the relationship between EL integrity and thickness for all donors examined in this investigation. Note that there is a strong relationship between these two parameters in all regions measured (exponential fit _R_2 = 0.8735).
Figure 7
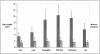
Top: Box and whiskers plots showing data for EL thickness (A, B) and integrity (C, D) derived from the macular (A, C) and extramacular (B, D) regions of unaffected donors <62 years, age-matched donors ≥62 years, donors with early-stage AMD, and donors with late-stage AMD (includes donors with GA, active CNVMs, and DSs). Median values are shown as bold red lines within each box), first and third quartile values are represented as blue and magenta, respectively, within the solid box, the minimum and maximum observation ranges are shown as whiskers and extreme values are represented as solitary, horizontal lines. One young donor with an extreme macular elastic layer thickness of 275 nm and one age-matched control donor with an extreme macular elastic layer thickness of 409 nm were omitted from graph A for scaling purposes.
Figure 8
Bottom: Box and whiskers plots showing data for EL thickness (A, B) and integrity (C, D) derived from the macular (A, C) and extramacular (B, D) regions of donors with early-stage AMD and subgroups of donors with late-stage AMD, including active CNVMs [CNV (act)], DSs (CNV/DS), and GA.
Figure 9
Transmission electron micrographs depicting the EL of Bruch’s membrane in eyes from a 82-year-old, age-matched control donor (A), an 84-year-old donor with early AMD (B), and an 83-year-old AMD donor with active CNV (C). The elastic layers are depicted with arrows and longest gaps in macular EL integrity (maximum gap lengths) for each donor are bracketed.
Figure 10
Bar graph depicting the average values for the largest gap length, or discontinuity, in the EL of donors with early-stage AMD and subgroups of donors with late-stage AMD (active CNVM, CNVM/DS, and GA). Significant differences in maximum gap length are noted between the both control groups (young and age-matched) and the early AMD, active CNVM, and CNVM/DS groups (all P < 0.0001), but not between the age-matched control group and the GA group (P = 0.11).
Figure 11
Models depicting possible scenarios that might lead to the enhanced susceptibility of the macula to CNVM formation (A) and CNVM recurrence (B). We have proposed that AMD-associated pathways, such as inflammation, that degrade or disrupt elastin might affect the barrier function of Bruch’s membrane in the macular region before that of the extramacular region because the elastic layer is the thinnest and most porous in this region (A and B, lane 1). The resulting breaches of Bruch’s membrane in the macula (asterisks; A and B, lane 2) might allow CNVMs to penetrate Bruch’s membrane and to grow into the subretinal and/or sub-RPE spaces. In treatments intended to destroy these neovessels, such as laser photocoagulation and photodynamic therapy (wavy lines; B, lane 3), the breached region of Bruch’s membrane might be replaced by fibrotic scar tissue (hatches; B, lane 4). Subsequently, the process of elastin degradation and neovascularization would continue at thinned, porous regions of the EL lying adjacent to that of the original vascular penetration (B, lanes 4 and 5). Ultimately, this process might continue until a scar formed over that portion of the macula (hatches; B, lane 6) where the EL was initially the thinnest and most porous.
Similar articles
- Iron accumulation in Bruch's membrane and melanosomes of donor eyes with age-related macular degeneration.
Biesemeier A, Yoeruek E, Eibl O, Schraermeyer U. Biesemeier A, et al. Exp Eye Res. 2015 Aug;137:39-49. doi: 10.1016/j.exer.2015.05.019. Epub 2015 May 28. Exp Eye Res. 2015. PMID: 26026877 - Characteristics of Drusen and Bruch's membrane in postmortem eyes with age-related macular degeneration.
Spraul CW, Grossniklaus HE. Spraul CW, et al. Arch Ophthalmol. 1997 Feb;115(2):267-73. doi: 10.1001/archopht.1997.01100150269022. Arch Ophthalmol. 1997. PMID: 9046265 - Maculoplasty for age-related macular degeneration: reengineering Bruch's membrane and the human macula.
Del Priore LV, Tezel TH, Kaplan HJ. Del Priore LV, et al. Prog Retin Eye Res. 2006 Nov;25(6):539-62. doi: 10.1016/j.preteyeres.2006.08.001. Epub 2006 Oct 30. Prog Retin Eye Res. 2006. PMID: 17071125 Review.
Cited by
- The retinal pigment epithelium: Development, injury responses, and regenerative potential in mammalian and non-mammalian systems.
George SM, Lu F, Rao M, Leach LL, Gross JM. George SM, et al. Prog Retin Eye Res. 2021 Nov;85:100969. doi: 10.1016/j.preteyeres.2021.100969. Epub 2021 Apr 23. Prog Retin Eye Res. 2021. PMID: 33901682 Free PMC article. Review. - Adhesion failures determine the pattern of choroidal neovascularization in the eye: a computer simulation study.
Shirinifard A, Glazier JA, Swat M, Gens JS, Family F, Jiang Y, Grossniklaus HE. Shirinifard A, et al. PLoS Comput Biol. 2012;8(5):e1002440. doi: 10.1371/journal.pcbi.1002440. Epub 2012 May 3. PLoS Comput Biol. 2012. PMID: 22570603 Free PMC article. - Localized Structural and Functional Deficits in a Nonhuman Primate Model of Outer Retinal Atrophy.
Liu YV, Konar G, Aziz K, Tun SBB, Hua CHE, Tan B, Tian J, Luu CD, Barathi VA, Singh MS. Liu YV, et al. Invest Ophthalmol Vis Sci. 2021 Oct 4;62(13):8. doi: 10.1167/iovs.62.13.8. Invest Ophthalmol Vis Sci. 2021. PMID: 34643661 Free PMC article. - Esterified cholesterol is highly localized to Bruch's membrane, as revealed by lipid histochemistry in wholemounts of human choroid.
Rudolf M, Curcio CA. Rudolf M, et al. J Histochem Cytochem. 2009 Aug;57(8):731-9. doi: 10.1369/jhc.2009.953448. Epub 2009 Apr 13. J Histochem Cytochem. 2009. PMID: 19365091 Free PMC article. - Increased choroidal neovascularization following laser induction in mice lacking lysyl oxidase-like 1.
Yu HG, Liu X, Kiss S, Connolly E, Gragoudas ES, Michaud NA, Bulgakov OV, Adamian M, DeAngelis MM, Miller JW, Li T, Kim IK. Yu HG, et al. Invest Ophthalmol Vis Sci. 2008 Jun;49(6):2599-605. doi: 10.1167/iovs.07-1508. Epub 2008 Feb 22. Invest Ophthalmol Vis Sci. 2008. PMID: 18296663 Free PMC article.
References
- Marshall J, Hussain A, Starita C, Moore D, Patmore A. Aging and Bruch’s membrane. Marmor M, Wolfensberger T, editors. New York: Oxford University Press,; The Retinal Pigment Epithelium. 1988:pp 669–692.
- Guymer R, Bird A. Bruch’s membrane, drusen, and age-related macular degeneration. Marmor M, Wolfensberger T, editors. New York: Oxford University Press,; The Retinal Pigment Epithelium. 1988:pp 693–705.
- Hogan M. Bruch’s membrane and disease of the macula. Trans Ophthalmol Soc UK. 1967;87:113–161. - PubMed
- Hogan M, Alvarado J. Studies on the human macula. Aging changes in Bruch’s membrane. Arch Ophthalmol. 1967;77:410–420. - PubMed
- Guymer R, Luthert P, Bird A. Changes in Bruch’s membrane and related structures with age. Prog Retinal Eye Res. 1999;18:59–90. - PubMed
Publication types
MeSH terms
Grants and funding
- T32 AG000214/AG/NIA NIH HHS/United States
- R01 EY011515/EY/NEI NIH HHS/United States
- EY11515/EY/NEI NIH HHS/United States
- EY014563/EY/NEI NIH HHS/United States
- R03 EY014563/EY/NEI NIH HHS/United States
- Z01 AG000214/ImNIH/Intramural NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous