Growth factor receptor binding protein 2-mediated recruitment of the RING domain of Cbl to the epidermal growth factor receptor is essential and sufficient to support receptor endocytosis - PubMed (original) (raw)
Growth factor receptor binding protein 2-mediated recruitment of the RING domain of Cbl to the epidermal growth factor receptor is essential and sufficient to support receptor endocytosis
Fangtian Huang et al. Mol Biol Cell. 2005 Mar.
Abstract
Knockdown of growth factor receptor binding protein 2 (Grb2) by RNA interference strongly inhibits clathrin-mediated endocytosis of the epidermal growth factor receptor (EGFR). To gain insights into the function of Grb2 in EGFR endocytosis, we have generated cell lines in which endogenous Grb2 was replaced by yellow fluorescent protein (YFP)-tagged Grb2 expressed at the physiological level. In these cells, Grb2-YFP fully reversed the inhibitory effect of Grb2 knockdown on EGFR endocytosis and, moreover, trafficked together with EGFR during endocytosis. Overexpression of Grb2-binding protein c-Cbl did not restore endocytosis in Grb2-depleted cells. However, EGFR endocytosis was rescued in Grb2-depleted cells by chimeric proteins consisting of the Src homology (SH) 2 domain of Grb2 fused to c-Cbl. The "knockdown and rescue" analysis revealed that the expression of Cbl-Grb2/SH2 fusions containing RING finger domain of Cbl restores normal ubiquitylation and internalization of the EGFR in the absence of Grb2, consistent with the important role of the RING domain in EGFR endocytosis. In contrast, the carboxy-terminal domain of Cbl, when attached to Grb2 SH2 domain, had 4 times smaller endocytosis-rescue effect compared with the RING-containing chimeras. Together, the data suggest that the interaction of Cbl carboxy terminus with CIN85 has a minor and a redundant role in EGFR internalization. We concluded that Grb2-mediated recruitment of the functional RING domain of Cbl to the EGFR is essential and sufficient to support receptor endocytosis.
Figures
Figure 1.
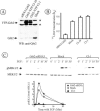
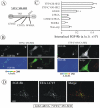
Expression of Grb2-YFP restores EGFR internalization and mitogen-activated protein kinase signaling in Grb2-depleted HeLa cells. (A) Western blot detection of Grb2 in HeLa cells transiently transfected with Grb2 siRNA (siRNA Grb2), transiently mock-transfected (Mock), and HeLa cell lines Cl-10 and Cl-1 stably expressing Grb2-YFP and U6/Grb2-siRNA plasmids. Asterisks show nonspecific bands. (B) 125I-EGF (1 ng/ml) internalization rate constant (_k_e) measured in cells described in A. The data represent mean values from three experiments (±SEM). (C) Cells described in A were serum starved overnight and treated with 1 ng/ml EGF at 37°C for the indicated times. Cell lysates were resolved by electrophoresis. Activated MEK1/2 was detected by blotting with antibodies to phosphorylated MEK1/2. The same blots were then reprobed with the antibody to total MEK1/2. The extent of MEK1/2 phosphorylation is expressed as a ratio of phosphorylated to total cellular MEK1/2.
Figure 2.
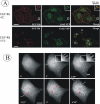
Visualization of Grb2-YFP in HeLa/Grb2-YFP Cl-1 cells. (A) Cl-1 cells were treated with 10 ng/ml EGF-Rh at 4°C for 1 h or with 2 ng/ml EGF-Rh at 37°C for 5 min and fixed. A z-stack of images was acquired through the Cy3 (red) and YFP (green) channels and deconvoluted. YFP and Cy3 images representing individual optical sections were merged after adjustment of both fluorescence signals to similar levels (Merge). Insets represent high-magnification images of the regions shown by white rectangles. In the merged inset image, YFP image was shifted approximately by 230 nm to the left relative to rhodamine images to clearly assess the colocalization of Grb2-YFP with EGF-Rh. n, cell nuclei. Bars, 10 μm. (B) Cells were incubated with 100 ng/ml EGF at 33°C, and images were acquired at 15-s intervals. This high concentration of EGF was used because of the significant dilution of EGF during perfusion of the microscope chamber. Times after EGF addition are indicated in the left bottom corner of the images. White arrows show Grb2-YFP localized to endocytic coats and forming vesicles at cell edges, whereas red arrows show Grb2-YFP localized in endosomes at some distance from cell edges or in the perinuclear area of the cell. The corresponding Quicktime movie is presented in Supplemental Materials. Bar, 10 μm.
Figure 3.
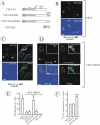
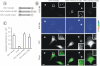
YFP-Cbl-SH2 chimera rescues EGFR internalization in HeLa cells depleted of Grb2. (A) Schematic representation of Cbl-Grb2 chimeric proteins fused to YFP. The YFP-Cbl-SH2 chimera consists of YFP, residues 1–906 of c-Cbl, and the SH2 domain of Grb2. YFP-Cbl-SH2/RA is a mutant YFP-Cbl-SH2 chimera with the substitution of Arg86 by alanine in the Grb2 SH2 domain. YFP-SH2 is the SH2 domain of Grb2 fused to YFP. (B–D) HeLa cells were mock transfected (B_)_ or transiently transfected (C and D) with Grb2 siRNA. The cells also were transfected with YFP-Cbl (C), YFP-Cbl-SH2 (D), or YFP-Cbl-SH2/RA (D, insets). The cells were incubated with 2 ng/ml EGF-Rh for 5 min at 37°C and fixed. A z-stack of optical sections was acquired through the Cy3 (red) and YFP (green) channels and deconvoluted. YFP and Cy3 images representing individual optical sections were merged after adjustment of the YFP signal (Merge). EGF-Rh images also are displayed in quantitative pseudocolor using the same scale for all images. A.l.u.f.i., arbitrary linear units of fluorescence intensity. White asterisks show positions of Grb2-depleted cells that do not express YFP fusion proteins (nonexp). Some of these cells can be seen by their autofluorescence in the YFP filter channel. Bars, 10 μm. (E) Quantification of the amount of internalized EGF-Rh from experiments performed as described in B–D. In experiments with all YFP-tagged proteins, the values of integrated intensity did not depend on the expression levels of these fusion proteins. The error bars represent SEs (n = 20). ***p < 0.0001 compared with cells depleted of Grb2 and not expressing YFP-tagged constructs (nonexp). (F) HeLa cells were transfected with control siRNA (mock) or Grb2 siRNA. The cells were also transfected with YFP-Cbl or YFP-Cbl-SH2 as described in D. Internalization rates of 125I-EGF were measured as in Figure 1B.
Figure 4.
EGF-Rh is internalized in AP-2–dependent manner and accumulates in early endosomes in cells depleted of Grb2 and expressing YFP-Cbl-SH2 chimera. (A) HeLa cells were mock transfected or transfected with Grb2 siRNA or μ2 siRNA. The cells were also transfected with YFP-Cbl-SH2 as described in Figure 3D. The incubations with EGF-Rh and quantitations of the amount of EGF-Rh in endosomes were performed as described in Figure 3, B–E. The error bars represent SEs (n = 10). ***p < 0.0001 compared with cells depleted of μ2. (B) HeLa cells were transfected with Grb2 siRNA and YFP-Cbl-SH2, incubated with EGF-Rh as described in D, fixed, permeabilized, and stained with a mAb to EEA.1 followed by secondary donkey anti-mouse IgG conjugated with Cy5. A z-stack of optical sections was acquired through the Cy3, Cy5, and YFP channels and deconvoluted. Cy5, YFP, and Cy3 images representing individual optical sections cells were merged (Merge). Yellow signifies colocalization of rhodamine and YFP labels; cyan of rhodamine and Cy5, and white of all three labels. Bar, 10 μm.
Figure 5.
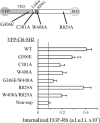
Point mutations in the YFP-Cbl-SH2 chimera affect endocytosis rescue capacity of the chimera in cells depleted of Grb2. HeLa cells were transiently transfected with Grb2 siRNA and either with YFP-Cbl-SH2 (WT) or its mutants schematically shown on the top. The cells were incubated with EGF-Rh, fixed, and imaged as described in Figure 3. The quantification of the amount of internalized EGF-Rh from experiments was performed as in Figure 3E. Cells transfected with Grb2 siRNA and not expressing YFP fusion proteins are designated as nonexp. In experiments with all YFP-tagged proteins, the values of integrated intensity did not depend on the expression levels of these fusion proteins. The error bars represent SEs (n = 20). **p < 0.002 compared with cells depleted of Grb2 and expressing YFP-Cbl-SH2 (WT). A.l.u.f.i., arbitrary linear units of fluorescence intensity.
Figure 6.
YFP-Cbl-SH2 chimeras bind EGFR and rescues ubiquitination of EGFR in Grb2-depleted cells. HeLa cells were transiently mock siRNA transfected or transfected with Grb2 siRNA. The cells also were mock DNA transfected or transfected with YFP-Cbl-SH2 chimera, mutant W408A of this chimera (see Figure 4), or YFP-Cbl. The cells were incubated with 20 ng/ml EGF for 2 min at 37°C and lysed. EGFR was immunoprecipitated (IP) with antibody 528. Immunoprecipitates and lysates were resolved on 7.5% SDS-PAGE and probed by Western blotting with antibody P4D1 to ubiquitin (A), GFP (B), antibody 2913 to EGFR (C), Grb2 (D), c-Cbl (E), and PP1 (F, nonspecific control). Asterisk in B marks a portion of the blot that was overexposed to demonstrate the presence of a small amount of YFP-Cbl in EGFR immunoprecipitates.
Figure 7.
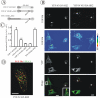
Grb2 chimera with amino terminus of Cbl restores EGFR internalization in Grb2-depleted cells. (A) Schematic representation of YFP-C'450-SH2 chimeric protein. YFP-C'450-SH2 contains c-Cbl fragment encompassing residues 1–450 flanked with YFP and the SH2 domain of Grb2. Mutations made in the construct are indicated. For example, YFP-C'450/CA-SH2 is a mutant of YFP-C'450-SH2 with the substitution of Cys381 by alanine. (B) HeLa cells were transfected with Grb2 siRNA and YFP-C'450-SH2, YFP-C'450/CA-SH2 or other mutants (our unpublished data). The cells were treated with EGF-Rh, fixed, and imaged as described in Figure 3. YFP and Cy3 images representing individual optical sections were merged after adjustment of the YFP signal (Merge). EGF-Rh images also are displayed in quantitative pseudocolor by using the same scale for both images. A.l.u.f.i., arbitrary linear units of fluorescence intensity. White asterisks show positions of Grb2-depleted cells that do not express YFP fusion proteins (nonexp). Bars, 10 μm. (C) Quantification of the amount of internalized EGF-Rh from experiments performed as described in B. In experiments with all YFP-tagged proteins, the values of integrated intensity did not depend on the expression levels of these fusion proteins. The error bars represent SEs (n = 15–20). *p < 0.05 compared with cells depleted of Grb2 and not expressing YFP-tagged constructs (nonexp). (D) HeLa cells transfected with Grb2 siRNA and YFP-C'450-SH2 were incubated with EGF-Rh, fixed, and processed for immunostaining with EEA.1 antibody as in Figure 3F. The z-stack of optical sections was acquired through the Cy3 (red), Cy5 (green), and YFP (our unpublished data) channels and deconvoluted. Cy5 and Cy3 images representing individual optical sections were merged (Merge). Yellow signifies colocalization of rhodamine and Cy5 labels. Bar, 10 μm.
Figure 8.
Chimera consisting of PLCγ1 SH2 domains and the amino terminus of Cbl restores EGFR internalization in Grb2-depleted cells. (A) Schematic representation of YFP-C'450-SH2 chimeric proteins. YFP-C'450-PLC.N/SH2 and YFP-C'450-PLC.NC/SH2 contain c-Cbl fragment encompassing residues 1–450 flanked with YFP and, respectively, the single N-SH2 domain or a tandem of two SH2 domains of PLCγ1. (B) HeLa cells were cotransfected with Grb2 siRNA and either YFP-C'450-SH2, YFP-C'450-PLC.N/SH2, or YFP-C'450-PLC.NC/SH2. The cells were treated with EGF-Rh, fixed, and imaged as described in Figure 3. YFP and Cy3 images representing individual optical sections were merged after adjustment of the YFP signal (Merge). EGF-Rh images also are displayed in quantitative pseudocolor by using the same scale for both images. Insets represent high-magnification images of the regions shown by white rectangles and demonstrate localization of the chimeric proteins in endosomes containing EGF-Rh. A.l.u.f.i., arbitrary linear units of fluorescence intensity. Bars, 10 μm. (C) Quantification of the amount of internalized EGF-Rh from experiments similar to those presented in B. The error bars represent SEs (n = 10).
Figure 9.
CIN85 interaction with Cbl is dispensable for EGFR internalization. (A) Schematic representation of Cbl-Grb2 chimeric proteins fused to YFP. The YFP-N′435-SH2 chimera consists of YFP, residues 435–906 of c-Cbl, and the SH2 domain of Grb2. YFP-N′435/RA-SH2 is a mutant YFP-N′435-SH2 chimera with the substitution of Arg829 (essential for CIN85 binding) to alanine in the c-Cbl fragment. YFP-Cbl/RA-SH2 contains YFP, full-length c-Cbl with the R829A mutation, and the SH2 domain of Grb2. (B) HeLa cells were transfected with Grb2 siRNA and either YFP-N′435-SH2 or YFP-N′435/RA-SH2. Cells were incubated with EGF-Rh, fixed, and imaged as described in Figure 3. YFP and Cy3 images representing individual optical sections were merged (Merge). EGF-Rh images also are displayed in quantitative pseudocolor using the same scale for both images. A.l.u.f.i., arbitrary linear units of fluorescence intensity. White asterisks show positions of Grb2-depleted cells that do not express YFP fusion proteins (nonexp). Inset represents high-magnification image of the region shown by white rectangles and demonstrate localization of the chimeric protein in endosomes containing EGF-Rh. Bars, 10 μm. (C) Quantification of the amount of internalized EGF-Rh from experiments similar to those presented in Figures 7B and 9B. In experiments with all YFP-tagged proteins, the values of integrated intensity did not depend on the expression levels of these fusion proteins. The error bars represent SEs (n = 20). ***p < 0.0001 compared with cells depleted of Grb2 and not expressing YFP-tagged constructs (nonexp). (D) HeLa cells transfected with Grb2 siRNA and YFP-N′435-SH2 were incubated with EGF-Rh for 15 min at 37°C, fixed, and processed for immunostaining with EEA.1 antibody. The z-stack of optical sections was acquired through the Cy3 (red), Cy5 (green) and YFP (our unpublished data) channels and deconvoluted. Cy5 and Cy3 images representing individual optical sections were merged (Merge). Yellow signifies colocalization of rhodamine and Cy5 labels. Bar, 10 μm.
Similar articles
- Ubiquitin ligase activity of c-Cbl guides the epidermal growth factor receptor into clathrin-coated pits by two distinct modes of Eps15 recruitment.
de Melker AA, van der Horst G, Borst J. de Melker AA, et al. J Biol Chem. 2004 Dec 31;279(53):55465-73. doi: 10.1074/jbc.M409765200. Epub 2004 Oct 1. J Biol Chem. 2004. PMID: 15465819 - Tyrosine phosphorylation of Cbl upon epidermal growth factor (EGF) stimulation and its association with EGF receptor and downstream signaling proteins.
Fukazawa T, Miyake S, Band V, Band H. Fukazawa T, et al. J Biol Chem. 1996 Jun 14;271(24):14554-9. doi: 10.1074/jbc.271.24.14554. J Biol Chem. 1996. PMID: 8662998 - Internalization of the epidermal growth factor receptor: role in signalling.
Sorkin A. Sorkin A. Biochem Soc Trans. 2001 Aug;29(Pt 4):480-4. doi: 10.1042/bst0290480. Biochem Soc Trans. 2001. PMID: 11498013 Review. - Endocytosis and intracellular trafficking of ErbBs.
Sorkin A, Goh LK. Sorkin A, et al. Exp Cell Res. 2008 Oct 15;314(17):3093-106. doi: 10.1016/j.yexcr.2008.08.013. Epub 2008 Aug 28. Exp Cell Res. 2008. PMID: 18793634 Free PMC article. Review.
Cited by
- Cbl and Cbl-b independently regulate EGFR through distinct receptor interaction modes.
Pinilla-Macua I, Sorkin A. Pinilla-Macua I, et al. Mol Biol Cell. 2023 Dec 1;34(13):ar134. doi: 10.1091/mbc.E23-02-0058. Epub 2023 Oct 30. Mol Biol Cell. 2023. PMID: 37903221 Free PMC article. - Endocytic downregulation of ErbB receptors: mechanisms and relevance in cancer.
Roepstorff K, Grøvdal L, Grandal M, Lerdrup M, van Deurs B. Roepstorff K, et al. Histochem Cell Biol. 2008 May;129(5):563-78. doi: 10.1007/s00418-008-0401-3. Epub 2008 Feb 21. Histochem Cell Biol. 2008. PMID: 18288481 Free PMC article. Review. - ARAP1 regulates endocytosis of EGFR.
Yoon HY, Lee JS, Randazzo PA. Yoon HY, et al. Traffic. 2008 Dec;9(12):2236-52. doi: 10.1111/j.1600-0854.2008.00839.x. Epub 2008 Oct 8. Traffic. 2008. PMID: 18939958 Free PMC article. - Mutational activation of ErbB family receptor tyrosine kinases: insights into mechanisms of signal transduction and tumorigenesis.
Riese DJ 2nd, Gallo RM, Settleman J. Riese DJ 2nd, et al. Bioessays. 2007 Jun;29(6):558-65. doi: 10.1002/bies.20582. Bioessays. 2007. PMID: 17508401 Free PMC article. Review. - Signaling-mediated control of ubiquitin ligases in endocytosis.
Polo S. Polo S. BMC Biol. 2012 Mar 15;10:25. doi: 10.1186/1741-7007-10-25. BMC Biol. 2012. PMID: 22420864 Free PMC article. Review.
References
- Ahn, S., Kim, J., Lucaveche, C. L., Reedy, M. C., Luttrell, L. M., Lefkowitz, R. J., and Daaka, Y. (2002). Src-dependent tyrosine phosphorylation regulates dynamin self-assembly and ligand-induced endocytosis of the EGF receptor. J. Biol. Chem. 277, 26642-26651. - PubMed
- Chang, C.-P., et al. (1993). Ligand-induced internalization of the EGF receptor is mediated by multiple endocytic codes analogous to the tyrosine motif found in constitutively internalized receptors. J. Biol. Chem. 268, 19312-19320. - PubMed
- Chattopadhyay, A., Vecchi, M., Ji, Q., Mernaugh, R., and Carpenter, G. (1999). The role of individual SH2 domains in mediating association of phospholipase C-gamma1 with the activated EGF receptor. J. Biol. Chem. 274, 26091-26097. - PubMed
- Chen, W. S., Lazar, C. S., Lund, K. A., Welsh, J. B., Chang, C. P., Walton, G. M., Der, C. J., Wiley, H. S., Gill, G. N., and Rosenfeld, M. G. (1989). Functional independence of the epidermal growth factor receptor from a domain required for ligand-induced internalization and calcium regulation. Cell 59, 33-43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous