BAFF is produced by astrocytes and up-regulated in multiple sclerosis lesions and primary central nervous system lymphoma - PubMed (original) (raw)
. 2005 Jan 17;201(2):195-200.
doi: 10.1084/jem.20041674. Epub 2005 Jan 10.
Diethilde Theil, Tobias Derfuss, Andreas Rosenwald, Frank Schrader, Camelia-Maria Monoranu, Susan L Kalled, Donna M Hess, Barbara Serafini, Francesca Aloisi, Hartmut Wekerle, Reinhard Hohlfeld, Edgar Meinl
Affiliations
- PMID: 15642740
- PMCID: PMC2212784
- DOI: 10.1084/jem.20041674
BAFF is produced by astrocytes and up-regulated in multiple sclerosis lesions and primary central nervous system lymphoma
Markus Krumbholz et al. J Exp Med. 2005.
Abstract
We report that B cell-activating factor of the tumor necrosis factor (TNF) family (BAFF) is expressed in the normal human brain at approximately 10% of that in lymphatic tissues (tonsils and adenoids) and is produced by astrocytes. BAFF was regularly detected by enzyme-linked immunosorbent assay in brain tissue lysates and in normal spinal fluid, and in astrocytes by double fluorescence microscopy. Cultured human astrocytes secreted functionally active BAFF after stimulation with interferon-gamma and TNF-alpha via a furin-like protease-dependent pathway. BAFF secretion per cell was manifold higher in activated astrocytes than in monocytes and macrophages. We studied brain lesions with B cell components, and found that in multiple sclerosis plaques, BAFF expression was strongly up-regulated to levels observed in lymphatic tissues. BAFF was localized in astrocytes close to BAFF-R-expressing immune cells. BAFF receptors were strongly expressed in situ in primary central nervous system (CNS) lymphomas. This paper identifies astrocytes as a nonimmune source of BAFF. CNS-produced BAFF may support B cell survival in inflammatory diseases and primary B cell lymphoma.
Figures
Figure 1.
BAFF transcripts in brain specimens and lymphatic tissue. RT-PCR detected full-length BAFF and the splice variant ΔBAFF in human CNS and lymphatic tissue. Genomic DNA (gDNA) and H2O served as controls.
Figure 2.
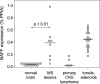
BAFF expression in normal CNS, MS lesions, and primary CNS lymphomas. Expression level of individual tissue samples was determined by quantitative PCR, and the median of each group is indicated. Mann-Whitney rank sum test was used for statistical analysis. Note that the BAFF levels in normal CNS and primary CNS lymphoma were not zero, but clearly detectable in each specimen with a mean of 0.034% PPIA.
Figure 3.
BAFF and BAFF-R–expressing cells in the normal CNS, MS, and primary CNS lymphoma. (A–D) BAFF in the normal CNS. (A) Molecular stratum and (B) granular stratum were from the cerebellum, (C and D) from the cerebrum. BAFF immunolocalized to scattered astrocytes in the perivascular area (A) and in the parenchyma (B–D). Similar staining patterns were observed with the two different BAFF-specific mAbs B1A3.2 (A–C) and A9C9.1 (D). (E–N) BAFF-specific astrocytes in different MS lesions. (E–G and M) Patient 1 (acute lesion); (H–L) patient 2 (chronic lesions); and (N) patient 3 (chronic lesion). BAFF-positive astrocytes are detected in the perivascular area (H–L) and in the parenchyma (H–J, M, and N). Double stainings are shown for GFAP (F and I, red), BAFF (E and H, green), and the overlays (G and J). Confocal microscopy (H–J) shows that BAFF-positive astrocytes form a network in the parenchyma and in the perivascular area. The two different BAFF-specific mAbs give similar staining patterns (K and L). (M) BAFF on astrocytes is demonstrated in the vicinity of infiltrating immune cells. The mAb B1A3.2 (E, G, H, J, K, M, N) and the mAb A9C9.1 (L) were applied. (O) A scattered cell in the germinal center of a tonsil is stained for BAFF (mAb B1A3.2). (P) BAFF-R expression on infiltrating immune cells within an acute MS lesion (patient 1). (Q) BAFF-R on the densely packed tumor cells on a primary CNS lymphoma. Sections were developed with diaminobenzidine that resulted in a brown color; nuclei were stained blue with hematoxylin (A–D and K–Q). Bars, 20 μm.
Figure 4.
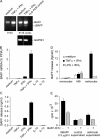
BAFF production by cultured astrocytes. (A) RT-PCR identified full-length BAFF and the splice variant ΔBAFF. To detect the constitutive expression of both isoforms and the induction by cytokine stimulation (25 h), two different cycle numbers were used as indicated. (B and C) Regulation by inflammatory cytokines. From the same stimulated astrocytes, RNA and cell culture supernatant were analyzed after 48 h by quantitative PCR (B) and ELISA (C). Error bars represent the SD. (D) Comparison of BAFF secretion by astrocytes, monocytes, and macrophages. After stimulation for 48 h, cell culture supernatants were analyzed by ELISA. The means of two experiments with monocytes, three with macrophages, and two with astrocytes are shown. Error bars represent SEM. Astrocyte stimulation with LPS + IFN-γ was not determined (ND) because a series of experiments had shown that these cultures do not respond to LPS, in agreement with the absence of myeloid cells in these cultures. (E) Functional activity of astrocyte-derived BAFF in a B cell proliferation assay. The concentrated astrocyte supernatant was added at a final dilution of 1:10 to the B cell culture. Medium processed in the same way as the astrocyte supernatant served as negative control and recombinant BAFF served as positive control. BAFF-R:Fc was used to block the BAFF effect. Error bars indicate the SD.
Figure 5.
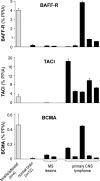
BAFF receptors in normal CNS, MS lesions, and primary CNS lymphomas. The expression level of BAFF-R, TACI, and BCMA was determined by quantitative PCR and is indicated as the percentage of the housekeeping gene PPIA. The mean values of 4 specimens from adenoids/tonsils and of 12 specimens from normal CNS are shown. Individual expression levels are shown for the analyzed pathological tissue specimens from MS lesions or primary CNS lymphomas. Error bars indicate the SD.
Similar articles
- Expression of B-cell-activating factor of the TNF family (BAFF) and its receptors in multiple sclerosis.
Thangarajh M, Gomes A, Masterman T, Hillert J, Hjelmström P. Thangarajh M, et al. J Neuroimmunol. 2004 Jul;152(1-2):183-90. doi: 10.1016/j.jneuroim.2004.03.017. J Neuroimmunol. 2004. PMID: 15223251 - B cell-activating factor of the tumor necrosis factor family (BAFF) is expressed under stimulation by interferon in salivary gland epithelial cells in primary Sjögren's syndrome.
Ittah M, Miceli-Richard C, Eric Gottenberg J, Lavie F, Lazure T, Ba N, Sellam J, Lepajolec C, Mariette X. Ittah M, et al. Arthritis Res Ther. 2006;8(2):R51. doi: 10.1186/ar1912. Epub 2006 Feb 28. Arthritis Res Ther. 2006. PMID: 16507175 Free PMC article. - Selectivity of BAFF/BLyS and APRIL for binding to the TNF family receptors BAFFR/BR3 and BCMA.
Day ES, Cachero TG, Qian F, Sun Y, Wen D, Pelletier M, Hsu YM, Whitty A. Day ES, et al. Biochemistry. 2005 Feb 15;44(6):1919-31. doi: 10.1021/bi048227k. Biochemistry. 2005. PMID: 15697217 - BAFF AND APRIL: a tutorial on B cell survival.
Mackay F, Schneider P, Rennert P, Browning J. Mackay F, et al. Annu Rev Immunol. 2003;21:231-64. doi: 10.1146/annurev.immunol.21.120601.141152. Epub 2001 Dec 19. Annu Rev Immunol. 2003. PMID: 12427767 Review. - The BAFF/APRIL system: an important player in systemic rheumatic diseases.
Mackay F, Sierro F, Grey ST, Gordon TP. Mackay F, et al. Curr Dir Autoimmun. 2005;8:243-65. doi: 10.1159/000082106. Curr Dir Autoimmun. 2005. PMID: 15564724 Review.
Cited by
- Proteomics Reveals Age as Major Modifier of Inflammatory CSF Signatures in Multiple Sclerosis.
Held F, Makarov C, Gasperi C, Flaskamp M, Grummel V, Berthele A, Hemmer B. Held F, et al. Neurol Neuroimmunol Neuroinflamm. 2025 Jan;12(1):e200322. doi: 10.1212/NXI.0000000000200322. Epub 2024 Nov 13. Neurol Neuroimmunol Neuroinflamm. 2025. PMID: 39536291 Free PMC article. - Serum BAFF levels are associated with the prognosis of idiopathic membranous nephropathy.
Li Z, Chen P, Zhang Y, Chen J, Zheng S, Li W, Tang L, Liu Y, Zhao N. Li Z, et al. Ren Fail. 2024 Dec;46(2):2391069. doi: 10.1080/0886022X.2024.2391069. Epub 2024 Aug 14. Ren Fail. 2024. PMID: 39143819 Free PMC article. - Enlarged perivascular spaces in alcohol-related brain damage induced by dyslipidemia.
Liu H, Meng L, Wang J, Qin C, Feng R, Chen Y, Chen P, Zhu Q, Ma M, Teng J, Ding X. Liu H, et al. J Cereb Blood Flow Metab. 2024 Oct;44(10):1867-1880. doi: 10.1177/0271678X241251570. Epub 2024 May 3. J Cereb Blood Flow Metab. 2024. PMID: 38700501 - B cells and the stressed brain: emerging evidence of neuroimmune interactions in the context of psychosocial stress and major depression.
Engler-Chiurazzi E. Engler-Chiurazzi E. Front Cell Neurosci. 2024 Apr 8;18:1360242. doi: 10.3389/fncel.2024.1360242. eCollection 2024. Front Cell Neurosci. 2024. PMID: 38650657 Free PMC article. Review. - Soluble CD27 is an intrathecal biomarker of T-cell-mediated lesion activity in multiple sclerosis.
Cencioni MT, Magliozzi R, Palmisano I, Suwan K, Mensi A, Fuentes-Font L, Villar LM, Fernández-Velasco JI, Migallón NV, Costa-Frossard L, Monreal E, Ali R, Romozzi M, Mazarakis N, Reynolds R, Nicholas R, Muraro PA. Cencioni MT, et al. J Neuroinflammation. 2024 Apr 12;21(1):91. doi: 10.1186/s12974-024-03077-9. J Neuroinflammation. 2024. PMID: 38609999 Free PMC article.
References
- Mackay, F., and S.G. Tangye. 2004. The role of the BAFF/APRIL system in B cell homeostasis and lymphoid cancers. Curr. Opin. Pharmacol. 4:347–354. - PubMed
- Nardelli, B., O. Belvedere, V. Roschke, P.A. Moore, H.S. Olsen, T.S. Migone, S. Sosnovtseva, J.A. Carrell, P. Feng, J.G. Giri, and D.M. Hilbert. 2001. Synthesis and release of B-lymphocyte stimulator from myeloid cells. Blood. 97:198–204. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical