The role of the hippocampus in the pathophysiology of major depression - PubMed (original) (raw)
Review
The role of the hippocampus in the pathophysiology of major depression
Stephanie Campbell et al. J Psychiatry Neurosci. 2004 Nov.
Abstract
Converging lines of research suggest that the hippocampal complex (HC) may have a role in the pathophysiology of major depressive disorder (MDD). Although postmortem studies show little cellular death in the HC of depressed patients, animal studies suggest that elevated glucocorticoid levels associated with MDD may negatively affect neurogenesis, cause excitotoxic damage or be associated with reduced levels of key neurotrophins in the HC. Antidepressant medications may counter these effects, having been shown to increase HC neurogenesis and levels of brain-derived neurotrophic factor in animal studies. Neuropsychological studies have identified deficits in hippocampus-dependent recollection memory that may not abate with euthymia, and such memory impairment has been the most reliably documented cognitive abnormality in patients with MDD. Finally, data from imaging studies suggest both structural changes in the volume of the HC and functional alterations in frontotemporal and limbic circuits that may be critical for mood regulation. The extent to which such functional and structural changes determine clinical outcome in MDD remains unknown; a related, but also currently unanswered, question is whether the changes in HC function and structure observed in MDD are preventable or modifiable with effective treatment for the depressive illness.
Figures
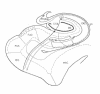
Fig. 1: Intrahippocampal pathways. The green line represents the direct intrahippocampal pathway from layer 3 of the entorhinal cortex (EC) to the CA1 region and then to the subiculum (SUB). From the subiculum, information can return to the entorhinal cortex or can enter the alveus (alv) and then the fimbria to influence neurotransmission in other cortical regions (red line). The afferent polysynaptic pathway is represented by the blue line. Axons from the entorhinal cortex enter the dentate gyrus (DG), and dendrites from the granule cells contact these glutamatergic axons. The mossy fibres of the granule cells project to the pyramidal neurons of the CA3 region, which then gives rise to efferent fibres to the alveus (in red) or Schaffer collateral fibres to the CA1 region. The pyramidal cells of the CA1 region, the primary output region of the cornu Ammonis, can project to either the subiculum (blue) or the alveus (red). Other regions displayed here include the perirhinal cortex (PRC), the parasubiculum (PaS) and the fusiform gyrus (FuG).
Fig. 2: Magnetic resonance spectroscopic images of the left hippocampus in a healthy control subject and in a patient with recurrent depression. The size of the difference shown here is unusually large, with most positive studies reporting a reduction in hippocampal complex (HC) volume of about 15% between cases and controls. Insert shows in blue the approximate sagittal level of the HC. Images were acquired on a 1.5-T GE Sigma Genesis–based EchoSpeed imager using previously published parameters. A: Sagittal view of the left HC, highlighted in red, of a healthy control subject whose left HC volume measured 3295 mm3. B: The patient whose left HC is represented here, with an HC volume of 2015 mm3, was of the same age and sex as the control subject but had a long history of recurrent depression.
Similar articles
- The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior.
Schmidt HD, Duman RS. Schmidt HD, et al. Behav Pharmacol. 2007 Sep;18(5-6):391-418. doi: 10.1097/FBP.0b013e3282ee2aa8. Behav Pharmacol. 2007. PMID: 17762509 Review. - Hippocampal volume, memory, and cortisol status in major depressive disorder: effects of treatment.
Vythilingam M, Vermetten E, Anderson GM, Luckenbaugh D, Anderson ER, Snow J, Staib LH, Charney DS, Bremner JD. Vythilingam M, et al. Biol Psychiatry. 2004 Jul 15;56(2):101-12. doi: 10.1016/j.biopsych.2004.04.002. Biol Psychiatry. 2004. PMID: 15231442 Clinical Trial. - Computational modeling and empirical studies of hippocampal neurogenesis-dependent memory: Effects of interference, stress and depression.
Becker S, Macqueen G, Wojtowicz JM. Becker S, et al. Brain Res. 2009 Nov 24;1299:45-54. doi: 10.1016/j.brainres.2009.07.095. Epub 2009 Aug 3. Brain Res. 2009. PMID: 19651106 - Critical role of brain-derived neurotrophic factor in mood disorders.
Hashimoto K, Shimizu E, Iyo M. Hashimoto K, et al. Brain Res Brain Res Rev. 2004 May;45(2):104-14. doi: 10.1016/j.brainresrev.2004.02.003. Brain Res Brain Res Rev. 2004. PMID: 15145621 Review. - Cognitive impairment in major depression and the mGlu2 receptor as a therapeutic target.
Goeldner C, Ballard TM, Knoflach F, Wichmann J, Gatti S, Umbricht D. Goeldner C, et al. Neuropharmacology. 2013 Jan;64:337-46. doi: 10.1016/j.neuropharm.2012.08.001. Epub 2012 Aug 23. Neuropharmacology. 2013. PMID: 22992331 Review.
Cited by
- Preliminary study of brain glucose metabolism changes in patients with lung cancer of different histological types.
Li WL, Fu C, Xuan A, Shi DP, Gao YJ, Zhang J, Xu JL. Li WL, et al. Chin Med J (Engl). 2015 Feb 5;128(3):301-4. doi: 10.4103/0366-6999.150089. Chin Med J (Engl). 2015. PMID: 25635423 Free PMC article. - Improving Outcome of Psychosocial Treatments by Enhancing Memory and Learning.
Harvey AG, Lee J, Williams J, Hollon SD, Walker MP, Thompson MA, Smith R. Harvey AG, et al. Perspect Psychol Sci. 2014 Mar;9(2):161-79. doi: 10.1177/1745691614521781. Perspect Psychol Sci. 2014. PMID: 25544856 Free PMC article. - Is cognitive dysfunction involved in difficult-to-treat depression? Characterizing resistance from a cognitive perspective.
López-Solà C, Subirà M, Serra-Blasco M, Vicent-Gil M, Navarra-Ventura G, Aguilar E, Acebillo S, Palao DJ, Cardoner N. López-Solà C, et al. Eur Psychiatry. 2020 Jun 23;63(1):e74. doi: 10.1192/j.eurpsy.2020.65. Eur Psychiatry. 2020. PMID: 32571441 Free PMC article. - Epigenetic and Neuronal Activity Markers Suggest the Recruitment of the Prefrontal Cortex and Hippocampus in the Three-Hit Model of Depression in Male PACAP Heterozygous Mice.
Gaszner T, Farkas J, Kun D, Ujvári B, Füredi N, Kovács LÁ, Hashimoto H, Reglődi D, Kormos V, Gaszner B. Gaszner T, et al. Int J Mol Sci. 2022 Oct 3;23(19):11739. doi: 10.3390/ijms231911739. Int J Mol Sci. 2022. PMID: 36233039 Free PMC article. - Telomerase dysregulation in the hippocampus of a rat model of depression: normalization by lithium.
Wei YB, Backlund L, Wegener G, Mathé AA, Lavebratt C. Wei YB, et al. Int J Neuropsychopharmacol. 2015 Jan 24;18(7):pyv002. doi: 10.1093/ijnp/pyv002. Int J Neuropsychopharmacol. 2015. PMID: 25618407 Free PMC article.
References
- Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Ann N Y Acad Sci 1999;877:614-37. - PubMed
- Duvernoy HM. The human hippocampus: functional anatomy, vascularization and serial sections with MRI. 2nd ed. Berlin: Springer-Verlag; 1998.
- Fritschy JM, Weinmann O, Wenzel A, Benke D. Synapse-specific localization of NMDA and GABA(A) receptor subunits revealed by antigen-retrieval immunohistochemistry. J Comp Neurol 1998;390:194-210. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources