AML1-ETO and C-KIT mutation/overexpression in t(8;21) leukemia: implication in stepwise leukemogenesis and response to Gleevec - PubMed (original) (raw)
. 2005 Jan 25;102(4):1104-9.
doi: 10.1073/pnas.0408831102. Epub 2005 Jan 13.
Guang-Biao Zhou, Tong Yin, Bing Chen, Jing-Yi Shi, Wen-Xue Liang, Xiao-Long Jin, Jian-Hua You, Guang Yang, Zhi-Xiang Shen, Jue Chen, Shu-Min Xiong, Guo-Qiang Chen, Feng Xu, Yi-Wei Liu, Zhu Chen, Sai-Juan Chen
Affiliations
- PMID: 15650049
- PMCID: PMC545849
- DOI: 10.1073/pnas.0408831102
AML1-ETO and C-KIT mutation/overexpression in t(8;21) leukemia: implication in stepwise leukemogenesis and response to Gleevec
Yue-Ying Wang et al. Proc Natl Acad Sci U S A. 2005.
Abstract
To explore the genetic abnormalities that cooperate with AML1-ETO (AE) fusion gene to cause acute myeloid leukemia (AML) with t(8;21), we screened a number of candidate genes and identified 11 types of mutations in C-KIT gene (mC-KIT), including 6 previously undescribed ones among 26 of 54 (48.1%) cases with t(8;21). To address a possible chronological order between AE and mC-KIT, we showed that, among patients with AE and mC-KIT, most leukemic cells at disease presentation harbored both genetic alteration, whereas in three such cases investigated during complete remission, only AE, but not mC-KIT, could be detected by allele-specific PCR. Therefore, mC-KIT should be a subsequent event on the basis of t(8;21). Furthermore, induced expression of AE in U937-A/E cells significantly up-regulated mRNA and protein levels of C-KIT. This may lead to an alternative way of C-KIT activation and may explain the significantly higher C-KIT expression in 81.3% of patients with t(8;21) than in patients with other leukemias. These data strongly suggest that t(8;21) AML follows a stepwise model in leukemogenesis, i.e., AE represents the first, fundamental genetic hit to initiate the disease, whereas activation of the C-KIT pathway may be a second but also crucial hit for the development of a full-blown leukemia. Additionally, Gleevec suppressed the C-KIT activity and induced proliferation inhibition and apoptosis in cells bearing C-KIT N822K mutation or overexpression, but not in cells with D816 mC-KIT. Gleevec also exerted a synergic effect in apoptosis induction with cytarabine, thus providing a potential therapeutic for t(8;21) leukemia.
Figures
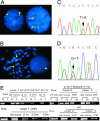
Fig. 1.
Coexistence of t(8;21) and mC-KITs in leukemic cells of de novo patients and persistence of AE but absence of mC-KIT in patients in CR. (A and B) Dual color FISH was performed, and 1,000 cells including interphase and metaphase were analyzed. The fusion signals (arrow) is seen in 96% in case 1 and 87.4% in case 2 of leukemic cells. Green signal, AML1 gene; orange signal, ETO gene. (C and D) Analysis of the peak height ratio of wild-type/mutant alleles in chromatogram of genomic DNA from leukemic cells of the two cases reveals that ≈85% in case 1 and 70% in case 2 of leukemic cells carried a mutant C-KIT allele. (E) The allele-specific RT-PCR is performed to detect the mutant alleles in three patients with t(8;21) leukemia at de novo and in remission. The primers used have an extra 2′-O,4′-_C_-methylene bridge at the ribose ring of nucleic acids at the 3′ end. The assays for Kasumi-1 (Kas) cells, which have a N822K (T → A) type mC-KIT, clearly show that these primers are sensitive and allele specific. At de novo, mC-KITs and AE are positive in the three patients, whereas the mC-KIT becomes undetectable and AE remains positive in CR.
Fig. 2.
Reduction of AE in CR and expression of C-KIT in t(8;21) leukemia. (A) AE expression in patients in CR decreased significantly compared to that in disease presentation. (B) Median of C-KIT log(CN) in t(8;21) and non-t(8;21) leukemias. (C and D) Immunohistochemical analysis of CD117 expression on bone marrow specimens of leukemia patients. (C) CD117-positive cells and their staining intensity are higher in patients with t(8;21) and mC-KIT than in those with t(8;21) and wtC-KIT, whereas the percentage of CD117-positive cells and their staining intensity are higher in patients with t(8;21) and wtC-KIT than in patients without t(8;21). (D) Accordingly, the immunoreactivity score (IRS) and staining intensity is higher in t(8;21) leukemia than in other hemopathies. (E) The C-KIT PTK activity is higher in t(8;21) leukemia than in other subtypes of leukemias.
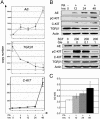
Fig. 3.
Induction of C-KIT expression by AE in vitro. (A) In U937-A/E cells treated with PA, the expression of AE at mRNA level increases gradually, whereas TGF_-β_1 is down-regulated at an early stage. The expression of C-KIT is up-regulated subtly at early stage (6–24 h) and dramatically at late stage (48 h). (B) At the protein level, the induced expression of AE down-regulates TGF-β1 and up-regulates phosphorylated (pC-KIT) and unphosphorylated C-KIT significantly (Upper). However, activation of C-KIT by SCF (μg/liter) do not modify AE and TGF-β1 expression (Lower). PA, ponasterone A (in μM); Gle, Gleevec (in μM). (C) The C-KIT PTK activity, which is reflected by absorbance at 450 nm on a spectrophotometer, is enhanced significantly 12 h after PA treatment.
Fig. 4.
Effects of Gleevec on t(8;21) leukemic cells. (A) Gleevec induces apoptosis of Kasumi-1 cells in a dose- and time-dependent manner. Gle, Gleevec, given in μM; time is given in hours. (B) Gleevec suppresses C-KIT PTK activity of the Kasumi-1 cell. (C_–_F) Effects of Gleevec and/or cytarabine (Ara-C, in μM) on primary leukemic cells from patients with t(8;21) leukemia. These effects are detected by annexin V assay (C_–_E) and analysis of morphological changes (F).
Similar articles
- The fusion protein AML1-ETO in acute myeloid leukemia with translocation t(8;21) induces c-jun protein expression via the proximal AP-1 site of the c-jun promoter in an indirect, JNK-dependent manner.
Elsässer A, Franzen M, Kohlmann A, Weisser M, Schnittger S, Schoch C, Reddy VA, Burel S, Zhang DE, Ueffing M, Tenen DG, Hiddemann W, Behre G. Elsässer A, et al. Oncogene. 2003 Aug 28;22(36):5646-57. doi: 10.1038/sj.onc.1206673. Oncogene. 2003. PMID: 12944913 - AML1-ETO9a is correlated with C-KIT overexpression/mutations and indicates poor disease outcome in t(8;21) acute myeloid leukemia-M2.
Jiao B, Wu CF, Liang Y, Chen HM, Xiong SM, Chen B, Shi JY, Wang YY, Wang JH, Chen Y, Li JM, Gu LJ, Tang JY, Shen ZX, Gu BW, Zhao WL, Chen Z, Chen SJ. Jiao B, et al. Leukemia. 2009 Sep;23(9):1598-604. doi: 10.1038/leu.2009.104. Epub 2009 May 21. Leukemia. 2009. PMID: 19458628 - A new D816 c-KIT gene mutation in refractory AML1-ETO leukemia.
Lasa A, Carricondo MT, Carnicer MJ, Perea G, Aventín A, Nomdedeu JF. Lasa A, et al. Haematologica. 2006 Sep;91(9):1283-4. Haematologica. 2006. PMID: 16956837 - AML1 and the AML1-ETO fusion protein in the pathogenesis of t(8;21) AML.
Licht JD. Licht JD. Oncogene. 2001 Sep 10;20(40):5660-79. doi: 10.1038/sj.onc.1204593. Oncogene. 2001. PMID: 11607817 Review. No abstract available. - The 8;21 translocation in leukemogenesis.
Peterson LF, Zhang DE. Peterson LF, et al. Oncogene. 2004 May 24;23(24):4255-62. doi: 10.1038/sj.onc.1207727. Oncogene. 2004. PMID: 15156181 Review.
Cited by
- Suppression of natural killer cells by sorafenib contributes to prometastatic effects in hepatocellular carcinoma.
Zhang QB, Sun HC, Zhang KZ, Jia QA, Bu Y, Wang M, Chai ZT, Zhang QB, Wang WQ, Kong LQ, Zhu XD, Lu L, Wu WZ, Wang L, Tang ZY. Zhang QB, et al. PLoS One. 2013;8(2):e55945. doi: 10.1371/journal.pone.0055945. Epub 2013 Feb 8. PLoS One. 2013. PMID: 23409093 Free PMC article. - Possible involvement of RasGRP4 in leukemogenesis.
Watanabe-Okochi N, Oki T, Komeno Y, Kato N, Yuji K, Ono R, Harada Y, Harada H, Hayashi Y, Nakajima H, Nosaka T, Kitaura J, Kitamura T. Watanabe-Okochi N, et al. Int J Hematol. 2009 May;89(4):470-481. doi: 10.1007/s12185-009-0299-0. Epub 2009 Apr 7. Int J Hematol. 2009. PMID: 19350351 - Roles of STAT3 and ZEB1 proteins in E-cadherin down-regulation and human colorectal cancer epithelial-mesenchymal transition.
Xiong H, Hong J, Du W, Lin YW, Ren LL, Wang YC, Su WY, Wang JL, Cui Y, Wang ZH, Fang JY. Xiong H, et al. J Biol Chem. 2012 Feb 17;287(8):5819-32. doi: 10.1074/jbc.M111.295964. Epub 2011 Dec 28. J Biol Chem. 2012. PMID: 22205702 Free PMC article. - [The study of clinical characteristics and prognosis of RUNX1-RUNX1T1 positive acute myeloid leukemia based on next-generation sequencing].
Wang YL, Gao SJ, Su L, Liu YJ, Zhang YW, Du YZ. Wang YL, et al. Zhonghua Xue Ye Xue Za Zhi. 2023 Oct 14;44(10):851-854. doi: 10.3760/cma.j.issn.0253-2727.2023.10.010. Zhonghua Xue Ye Xue Za Zhi. 2023. PMID: 38049338 Free PMC article. Chinese. No abstract available. - Prognostic Factors in Acute Myeloid Leukemia with t(8;21)/AML1-ETO: Strategies to Define High-Risk Patients.
Wang J, Gao N, Wang X, Yu W, Li A. Wang J, et al. Indian J Hematol Blood Transfus. 2022 Oct;38(4):631-637. doi: 10.1007/s12288-021-01507-9. Epub 2021 Dec 1. Indian J Hematol Blood Transfus. 2022. PMID: 36258727 Free PMC article.
References
- Look, A. T. (1997) Science 278, 1059–1064. - PubMed
- Reilly, J. T. (2002) Br. J. Haematol. 116, 744–757. - PubMed
- Kelly, L. M. & Gilliland, D. G. (2002) Annu. Rev. Genomics Hum. Genet. 3, 179–198. - PubMed
- Peterson, L. F. & Zhang, D. E. (2004) Oncogene 23, 4255–4262. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical