Update on human herpesvirus 6 biology, clinical features, and therapy - PubMed (original) (raw)
Review
Update on human herpesvirus 6 biology, clinical features, and therapy
Leen De Bolle et al. Clin Microbiol Rev. 2005 Jan.
Abstract
Human herpesvirus 6 (HHV-6) is a betaherpesvirus that is closely related to human cytomegalovirus. It was discovered in 1986, and HHV-6 literature has expanded considerably in the past 10 years. We here present an up-to-date and complete overview of the recent developments concerning HHV-6 biological features, clinical associations, and therapeutic approaches. HHV-6 gene expression regulation and gene products have been systematically characterized, and the multiple interactions between HHV-6 and the host immune system have been explored. Moreover, the discovery of the cellular receptor for HHV-6, CD46, has shed a new light on HHV-6 cell tropism. Furthermore, the in vitro interactions between HHV-6 and other viruses, particularly human immunodeficiency virus, and their relevance for the in vivo situation are discussed, as well as the transactivating capacities of several HHV-6 proteins. The insight into the clinical spectrum of HHV-6 is still evolving and, apart from being recognized as a major pathogen in transplant recipients (as exemplified by the rising number of prospective clinical studies), its role in central nervous system disease has become increasingly apparent. Finally, we present an overview of therapeutic options for HHV-6 therapy (including modes of action and resistance mechanisms).
Figures
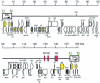
FIG. 1.
Schematic representation of the HHV-6B genomic organization. The terminal direct repeats (DRL and DRR) are boxed, and the intermediate repeats (R1, R2, and R3) are shown as red boxes. Telomeric sequences (T1 and T2) are denoted as green bars. The origin of lytic replication (Ori) is indicated by an asterisk. Protein coding regions are represented by open arrows; arrows colored yellow denote genes belonging to the HCMV US22 gene family. GCR, G-protein-coupled receptor; Ig, immunoglobulin superfamily; RR, ribonucleotide reductase; mCP, minor capsid protein; CA, capsid assembly protein; Teg, large tegument protein; Pol, DNA polymerase; tp, transport protein; mDBP, major single-stranded DNA binding protein; TA, conserved herpesvirus transactivator; dUT, dUTPase; Pts, protease/assembly protein; MCP, major capsid protein; PT, phosphotransferase; Exo, exonuclease; OBP, origin binding protein; Hel, helicase; UDG, uracil-DNA glycosylase; Che, chemokine; AAV/rep, adeno-associated virus-2 replication protein homolog. Reproduced from Isegawa et al. (183) with permission of the publisher.
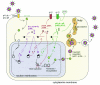
FIG. 2.
Schematic representation of the HHV-6 lytic replication cycle. The successive events of entry, replication, maturation, and egress are described in detail in “Replication cycle” in the text.
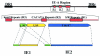
FIG. 3.
Schematic diagram of the HHV-6A IE-A region. The region encodes two proteins (termed IE1 and IE2), which correspond to the open reading frames U90-89 and U90-86/87, respectively. Reproduced from reference with permission of the publisher.
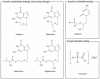
FIG. 4.
Chemical structures of clinically used anti-HHV-6 compounds.
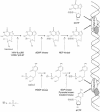
FIG. 5.
Metabolic activation of acyclic nucleoside analogs (exemplified by ganciclovir) and acyclic nucleoside phosphonates (represented by cidofovir). Abbreviations are explained in the text in “Ganciclovir and acyclovir” and “Cidofovir.”
FIG. 6.
ClustalW alignment of the HHV-6 pU69 and HCMV pUL97 protein sequences. The overall identity between HHV-6 GS and Z29 pU69 proteins is 94%. Identical residues are indicated by an asterisk. The colons and periods represent strong and weak functional similarities, respectively. Published mutations conferring ganciclovir phenotypic resistance (74, 277, 356) are boxed. Conserved domains are shaded. Amino acid residues that are highly conserved in protein kinases are highlighted within conserved domains. The GXGXXG motif thus indicated in domains I and II is the putative nucleotide binding site. The (A)ACR(AL) motif at amino acid positions 590 to 595 in pUL97 is essential for ganciclovir phosphorylation (381) and is among protein kinases unique to HHV-6 and HCMV.
FIG. 7.
Schematic representation of HCMV (A) and HHV-6 (B) DNA polymerases. Conserved regions are boxed. Functional domains are indicated by arrows. Bars indicate codons mapped to resistance to ganciclovir (GCV), cidofovir (CDV), and foscarnet (PFA). Regions showing variation in drug-sensitive isolates (Var) are indicated by shaded boxes. APB, accessory protein binding.
Similar articles
- Chromosomally integrated human herpesvirus-6 in transplant recipients.
Lee SO, Brown RA, Razonable RR. Lee SO, et al. Transpl Infect Dis. 2012 Aug;14(4):346-54. doi: 10.1111/j.1399-3062.2011.00715.x. Epub 2012 Feb 9. Transpl Infect Dis. 2012. PMID: 22321264 Review. - Human herpesvirus 6: molecular biology and clinical features.
Dockrell DH. Dockrell DH. J Med Microbiol. 2003 Jan;52(Pt 1):5-18. doi: 10.1099/jmm.0.05074-0. J Med Microbiol. 2003. PMID: 12488560 Review. - [Human herpesvirus type 6 and type 7 in transplant recipients].
Benito N, Moreno A, Pumarola T, Marcos MA. Benito N, et al. Enferm Infecc Microbiol Clin. 2003 Oct;21(8):424-32. doi: 10.1016/s0213-005x(03)72980-2. Enferm Infecc Microbiol Clin. 2003. PMID: 14525708 Review. Spanish. - Molecular biology and clinical associations of Roseoloviruses human herpesvirus 6 and human herpesvirus 7.
Caselli E, Di Luca D. Caselli E, et al. New Microbiol. 2007 Jul;30(3):173-87. New Microbiol. 2007. PMID: 17802896 Review. - A Murine Herpesvirus Closely Related to Ubiquitous Human Herpesviruses Causes T-Cell Depletion.
Patel SJ, Zhao G, Penna VR, Park E, Lauron EJ, Harvey IB, Beatty WL, Plougastel-Douglas B, Poursine-Laurent J, Fremont DH, Wang D, Yokoyama WM. Patel SJ, et al. J Virol. 2017 Apr 13;91(9):e02463-16. doi: 10.1128/JVI.02463-16. Print 2017 May 1. J Virol. 2017. PMID: 28179532 Free PMC article.
Cited by
- Viruses in glioblastoma: an update on evidence and clinical trials.
Gunasegaran B, Ashley CL, Marsh-Wakefield F, Guillemin GJ, Heng B. Gunasegaran B, et al. BJC Rep. 2024 Apr 19;2(1):33. doi: 10.1038/s44276-024-00051-z. BJC Rep. 2024. PMID: 39516641 Free PMC article. Review. - Human herpesvirus 6 (HHV-6) encephalitis secondary to chimeric antigen receptor (CAR)-T cell therapy.
Yi F, Qin N, Wang L. Yi F, et al. Neurol Sci. 2024 Nov 5. doi: 10.1007/s10072-024-07860-7. Online ahead of print. Neurol Sci. 2024. PMID: 39499455 - Acute Heart Failure in a Young Patient Treated in ICU-Diagnostic Pitfalls.
Surówka Ł, Andruszkiewicz P, Budnik M, Kowalik R, Milner A, Zawadka M. Surówka Ł, et al. Clin Pract. 2024 Sep 24;14(5):1953-1959. doi: 10.3390/clinpract14050155. Clin Pract. 2024. PMID: 39451870 Free PMC article. - Diagnostic Dilemmas: A Review of Reported Cases of Human Herpesvirus 6 Encephalitis in Immunocompetent Adults.
Webb G, Leong MYM, Bishop E, Sehu M. Webb G, et al. Open Forum Infect Dis. 2024 Sep 4;11(9):ofae501. doi: 10.1093/ofid/ofae501. eCollection 2024 Sep. Open Forum Infect Dis. 2024. PMID: 39301106 Free PMC article. Review.
References
- Ablashi, D. V., N. Balachandran, S. F. Josephs, C. L. Hung, G. R. Krueger, B. Kramarsky, S. Z. Salahuddin, and R. C. Gallo. 1991. Genomic polymorphism, growth properties, and immunologic variations in human herpesvirus-6 isolates. Virology 184:545-552. - PubMed
- Acott, P. D., S. H. Lee, H. Bitter-Suermann, J. G. Lawen, and J. F. Crocker. 1996. Infection concomitant with pediatric renal allograft rejection. Transplantation 62:689-691. - PubMed
- Adams, O., C. Krempe, G. Kogler, P. Wernet, and A. Scheid. 1998. Congenital infections with human herpesvirus 6. J. Infect. Dis. 178:544-546. - PubMed
- Agut, H., J. T. Aubin, and J. M. Huraux. 1991. Homogeneous susceptibility of distinct human herpesvirus 6 strains to antivirals in vitro. J. Infect. Dis. 163:1382-1383. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous