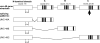The composition of the GABA receptor at the Caenorhabditis elegans neuromuscular junction - PubMed (original) (raw)
The composition of the GABA receptor at the Caenorhabditis elegans neuromuscular junction
Bruce A Bamber et al. Br J Pharmacol. 2005 Feb.
Abstract
1. The unc-49 gene of the nematode Caenorhabditis elegans encodes three gamma-aminobutyric acid type A (GABA(A)) receptor subunits. Two of these, UNC-49B and UNC-49C, are expressed at high abundance and co-localize at the neuromuscular junction. 2. The UNC-49B subunit is sufficient to form a GABA(A) receptor in vitro and in vivo. Furthermore, all loss-of-function unc-49 alleles lack functional UNC-49B. No mutations specifically inactivate UNC-49C. Thus, UNC-49C appears to be dispensable for receptor function; however, UNC-49C has been conserved among different nematode species, suggesting it plays a necessary role. 3. To ascertain whether UNC-49C is part of the GABA(A) receptor in vivo, we performed patch-clamp electrophysiology on C. elegans muscle cells. Sensitivity to GABA, and to the antagonists picrotoxin and pregnenolone sulfate, matched the UNC-49B/C heteromer rather than the UNC-49B homomer, for both exogenous and synaptically-released GABA. 4. The synaptic localization of UNC-49C requires the presence of UNC-49B, indicative of a physical association between the two subunits in vivo. Thus, the in vivo receptor is an UNC-49B/C heteromer. 5. UNC-49C plays a negative modulatory role. Using the rapid ligand-exchange technique in vitro, we determined that UNC-49C causes accelerated receptor desensitization. Previously, UNC-49C was shown to reduce single-channel conductance in UNC-49B/C heteromers. Thus, the function of UNC-49B is to provide GABA responsiveness and localization to synapses, while the function of UNC-49C is to negatively modulate receptor function and precisely shape inhibitory postsynaptic currents.
Figures
Figure 1
Summary of unc-49 gene structure and mRNA splicing. ‘c–c' refers to the conserved cysteine loop. Vertical bars represent transmembrane domains. Arrow indicates location of the deletion and frameshift mutations that eliminate the UNC-49C subunit in the unc-49 rescuing transgene, used to construct the UNC-49B transgenic strain and the UNC-49B-GFPΔC strain. Exons encoding the N-terminal domain are spliced to one of three alternative sets of exons (A, B, and C) encoding carboxy-terminal GABA binding and transmembrane domains, to produce the UNC-49A, UNC-49B, and UNC-49C cDNAs.
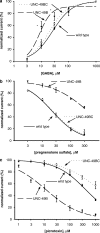
Figure 2
Agonist and antagonist sensitivity of C. elegans muscle cells. (a) GABA concentration–response curves for wild-type worms, UNC-49BC transgenic worms, and UNC-49B transgenic worms. Asterisks mark data points that are significantly different (P<0.05) between UNC-49B, and both the wild type and UNC-49BC. (b) Concentration–response curves for PS inhibition of currents evoked by 300 μ
M
GABA. (c) Concentration–response curves for PTX inhibition of currents evoked by 30 μ
M
GABA. Error bars are s.e.m.
Figure 3
Antagonist pharmacology of the synaptic GABAA receptor in C. elegans muscle. Acetylcholine minis were eliminated by bath application of
D
-tubocurare (500 μ
M
). (a) Traces of GABA minis recorded in the absence (left panel) and presence (middle panel) of 300 μ
M
PS. Plot of relative frequencies of GABA minis in the absence and presence of PS (right panel). (b) GABA minis recorded in the absence (left panel) and presence (middle panel) of 1000 μ
M
PTX. Plot of relative frequencies of GABA minis in the absence and presence of PTX (right panel). The genotype of the worm strain is indicated above each trace. PS is pregnenolone sulfate, PTX is picrotoxin. Error bars are s.e.m.
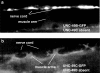
Figure 4
Synaptic localization of UNC-49C-GFP depends on UNC-49B. (a) Punctate nerve cord fluorescence is observed in worms expressing UNC-49B-GFP in the absence of UNC-49C. (b) No nerve cord-associated fluorescence is observed in worms expressing UNC-49C-GFP in the absence of UNC-49B. Fluorescence is observed on muscle arms and muscle cell membranes. The UNC-49C-GFP construct is localized to synapses in the wild type, which expresses UNC-49B (Bamber et al., 1999).
Figure 5
UNC-49C accelerates desensitization. (a) Outside-out patches pulled from HEK 293 cells expressing UNC-49B homomers (top trace) or UNC-49B/C heteromers (middle trace) were exposed to 10 m
M
GABA for 500 ms using the rapid ligand exchange technique. The line above each trace is the corresponding open tip junction potential, indicating when GABA was applied. Accelerated desensitization of UNC-49B/C heteromers is evident when the two traces are normalized and superimposed (lower panel). (b) Desensitization time constants for UNC-49B homomers and UNC-49B/C heteromers _n_=8 and 10, for UNC-49B homomers and UNC-49B/C heteromers, respectively. _τ_w, _τ_s, and _τ_f are the weighted, slow, and fast decay time constants, respectively. Error bars are s.e.m., asterisk indicates P<0.05 (Student's _t_-test).
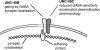
Figure 6
Model of interactions at the synapse. UNC-49B is linked to the cytoskeleton by a putative receptor tethering protein that binds to the subunit intracellular domain, and UNC-49C becomes localized to the synapse by physically associating with UNC-49B. The functional role of each subunit is indicated.
Similar articles
- The Caenorhabditis elegans unc-49 locus encodes multiple subunits of a heteromultimeric GABA receptor.
Bamber BA, Beg AA, Twyman RE, Jorgensen EM. Bamber BA, et al. J Neurosci. 1999 Jul 1;19(13):5348-59. doi: 10.1523/JNEUROSCI.19-13-05348.1999. J Neurosci. 1999. PMID: 10377345 Free PMC article. - An UNC-49 GABA receptor subunit from the parasitic nematode Haemonchus contortus is associated with enhanced GABA sensitivity in nematode heteromeric channels.
Siddiqui SZ, Brown DD, Rao VT, Forrester SG. Siddiqui SZ, et al. J Neurochem. 2010 Jun;113(5):1113-22. doi: 10.1111/j.1471-4159.2010.06651.x. Epub 2010 Feb 23. J Neurochem. 2010. PMID: 20180830 - Pharmacological characterization of the homomeric and heteromeric UNC-49 GABA receptors in C. elegans.
Bamber BA, Twyman RE, Jorgensen EM. Bamber BA, et al. Br J Pharmacol. 2003 Mar;138(5):883-93. doi: 10.1038/sj.bjp.0705119. Br J Pharmacol. 2003. PMID: 12642390 Free PMC article. - Alternative splicing in C. elegans.
Zahler AM. Zahler AM. WormBook. 2005 Sep 26:1-13. doi: 10.1895/wormbook.1.31.1. WormBook. 2005. PMID: 18050427 Free PMC article. Review. - Shaping cellular form and function by autophagy.
Bamber BA, Rowland AM. Bamber BA, et al. Autophagy. 2006 Jul-Sep;2(3):247-9. doi: 10.4161/auto.2746. Epub 2006 Jul 29. Autophagy. 2006. PMID: 16874044 Review.
Cited by
- A C. elegans genome-wide RNAi screen for altered levamisole sensitivity identifies genes required for muscle function.
Chaya T, Patel S, Smith EM, Lam A, Miller EN, Clupper M, Kervin K, Tanis JE. Chaya T, et al. G3 (Bethesda). 2021 Apr 15;11(4):jkab047. doi: 10.1093/g3journal/jkab047. G3 (Bethesda). 2021. PMID: 33713125 Free PMC article. - Electrophysiological methods for Caenorhabditis elegans neurobiology.
Goodman MB, Lindsay TH, Lockery SR, Richmond JE. Goodman MB, et al. Methods Cell Biol. 2012;107:409-36. doi: 10.1016/B978-0-12-394620-1.00014-X. Methods Cell Biol. 2012. PMID: 22226532 Free PMC article. - A molecular characterization of the agonist binding site of a nematode cys-loop GABA receptor.
Kaji MD, Kwaka A, Callanan MK, Nusrat H, Desaulniers JP, Forrester SG. Kaji MD, et al. Br J Pharmacol. 2015 Aug;172(15):3737-47. doi: 10.1111/bph.13158. Epub 2015 May 19. Br J Pharmacol. 2015. PMID: 25850584 Free PMC article. - Ion-channels on parasite muscle: pharmacology and physiology.
Robertson AP, Martin RJ. Robertson AP, et al. Invert Neurosci. 2007 Dec;7(4):209-17. doi: 10.1007/s10158-007-0059-x. Epub 2007 Nov 13. Invert Neurosci. 2007. PMID: 17999098 Review. - Graded synaptic transmission at the Caenorhabditis elegans neuromuscular junction.
Liu Q, Hollopeter G, Jorgensen EM. Liu Q, et al. Proc Natl Acad Sci U S A. 2009 Jun 30;106(26):10823-8. doi: 10.1073/pnas.0903570106. Epub 2009 Jun 15. Proc Natl Acad Sci U S A. 2009. PMID: 19528650 Free PMC article.
References
- ESSRICH C., LOREZ M., BENSON J.A., FRITSCHY J.M., LUSCHER B. Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat. Neurosci. 1998;1:563–571. - PubMed
- FISHER J.L., ZHANG J., MACDONALD R.L. The role of alpha1 and alpha6 subtype amino-terminal domains in allosteric regulation of gamma-aminobutyric acid A receptors. Mol. Pharmacol. 1997;52:714–724. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources