Tumour-derived exosomes and their role in cancer-associated T-cell signalling defects - PubMed (original) (raw)
Comparative Study
Tumour-derived exosomes and their role in cancer-associated T-cell signalling defects
D D Taylor et al. Br J Cancer. 2005.
Abstract
Dendritic and lymphoid 'exosomes' regulate immune activation. Tumours release membranous material mimicking these 'exosomes,' resulting in deletion of reactive lymphocytes. Tumour-derived 'exosomes' have recently been explored as vaccines, without analysis of their immunologic consequences. This investigation examines the composition of tumour-derived 'exosomes' and their effects on T lymphocytes. Membranous materials were isolated from ascites of ovarian cancer patients (n=6) and Western immunoblotting was performed for markers associated with 'exosomes.' Using cultured T cells, 'exosomes' were evaluated for suppression of CD3-zeta and JAK 3 expressions and induction of apoptosis, measured by DNA fragmentation. 'Exosome' components mediating suppression of CD3-zeta were isolated by continuous eluting electrophoresis and examined by Western immunoblotting. 'Exosomes' were shown to be identical with previously characterised shed membrane vesicles by protein staining and TSG101 expression. 'Exosomes' expressed class I MHC, placental alkaline phosphatase, B23/nucleophosmin, and FasL. 'Exosomes' suppressed expression of T-cell activation signalling components, CD3-zeta and JAK 3 and induced apoptosis. CD3-zeta suppression was mediated by two components: 26 and 42 kDa. Only the 42 kDa component reacted with anti-FasL antibody. These results indicate that, while 'exosomes' express tumour antigens, leading to their proposed utility as tumour vaccines, they also can suppress T-cell signalling molecules and induce apoptosis.
Figures
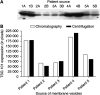
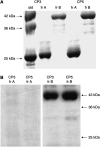
Figure 1
SDS–PAGE separation of chromatographically isolated membrane vesicles (designated lanes A) and centrifugally isolated ‘exosomes’ (designated lanes B) from the ascites of the same ovarian cancer patients, visualised by silver staining.
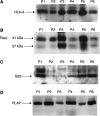
Figure 2
(A) Representative Western immunoblots of SDS–PAGE separation of chromatographically isolated membrane vesicles (designated lanes A) and centrifugally isolated ‘exosomes’ (designated lanes B) from the ascites of the same ovarian cancer patients, incubated with anti-TSG101 antibody and visualised by ECL. (B) Densitometric analysis of TSG101 expression on these membrane vesicle isolates.
Figure 3
Western immunoblots demonstrating presence of HLA-A, Fas ligand, B23/nucleophosmin, and placental alkaline phosphatase on centrifugally isolated ‘exosomes’ from the ascites of ovarian cancer patients.
Figure 4
(A) Western immunoblots indicating the expression of CD3-ζ protein and JAK 3 by Jurkat cells, following incubation with 400 _μ_g ml−1 of the centrifugally isolated ‘exosomes’ or analogous gradient material (Control, C) for 48 h. (B) Densitometric quantitation of CD3-ζ and JAK 3 expression by treated Jurkat cells.
Figure 5
Induction of apoptosis in Jurkat T cells by 400 _μ_g ml−1 of the centrifugally isolated ‘exosomes’ vs the analogous fraction from control female controls for 24 h, as defined by DNA fragmentation.
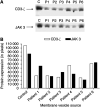
Figure 6
Identification of the ‘exosomes’-associated inhibitors of CD3-ζ expression. ‘Exosomes’ were fractionated by continuously eluting electrophoresis and fractions were subsequently assayed for ζ suppression in a Jurkat bioassay. The fractions suppressing ζ expression were examined by SDS–PAGE with silver staining (A). Western immunoblotting of the two fractions with anti-FasL demonstrated reactivity with the 42 kDa component, but not with the 26 kDa (B).
Similar articles
- Pregnancy-associated exosomes and their modulation of T cell signaling.
Taylor DD, Akyol S, Gercel-Taylor C. Taylor DD, et al. J Immunol. 2006 Feb 1;176(3):1534-42. doi: 10.4049/jimmunol.176.3.1534. J Immunol. 2006. PMID: 16424182 Retracted. - T-cell apoptosis and suppression of T-cell receptor/CD3-zeta by Fas ligand-containing membrane vesicles shed from ovarian tumors.
Taylor DD, Gerçel-Taylor C, Lyons KS, Stanson J, Whiteside TL. Taylor DD, et al. Clin Cancer Res. 2003 Nov 1;9(14):5113-9. Clin Cancer Res. 2003. PMID: 14613988 - Interleukin-12-anchored exosomes increase cytotoxicity of T lymphocytes by reversing the JAK/STAT pathway impaired by tumor-derived exosomes.
Zhang Y, Wu XH, Luo CL, Zhang JM, He BC, Chen G. Zhang Y, et al. Int J Mol Med. 2010 May;25(5):695-700. doi: 10.3892/ijmm_00000393. Int J Mol Med. 2010. PMID: 20372811 - The potential of exosomes in immunotherapy.
Chaput N, Taïeb J, André F, Zitvogel L. Chaput N, et al. Expert Opin Biol Ther. 2005 Jun;5(6):737-47. doi: 10.1517/14712598.5.6.737. Expert Opin Biol Ther. 2005. PMID: 15952905 Review. - Tumour-released exosomes and their implications in cancer immunity.
Iero M, Valenti R, Huber V, Filipazzi P, Parmiani G, Fais S, Rivoltini L. Iero M, et al. Cell Death Differ. 2008 Jan;15(1):80-8. doi: 10.1038/sj.cdd.4402237. Epub 2007 Oct 12. Cell Death Differ. 2008. PMID: 17932500 Review.
Cited by
- Targeting microRNAs as key modulators of tumor immune response.
Paladini L, Fabris L, Bottai G, Raschioni C, Calin GA, Santarpia L. Paladini L, et al. J Exp Clin Cancer Res. 2016 Jun 27;35:103. doi: 10.1186/s13046-016-0375-2. J Exp Clin Cancer Res. 2016. PMID: 27349385 Free PMC article. Review. - NKG2D-mediated cytotoxicity improves after primary surgery for high-grade serous ovarian cancer.
Israelsson P, Björk E, Nagaev I, Nagaeva O, Lundin E, Mincheva-Nilsson L, Ottander U. Israelsson P, et al. Am J Reprod Immunol. 2023 Jan;89(1):e13647. doi: 10.1111/aji.13647. Epub 2022 Nov 17. Am J Reprod Immunol. 2023. PMID: 36335434 Free PMC article. - The Development of an Angiogenic Protein "Signature" in Ovarian Cancer Ascites as a Tool for Biologic and Prognostic Profiling.
Trachana SP, Pilalis E, Gavalas NG, Tzannis K, Papadodima O, Liontos M, Rodolakis A, Vlachos G, Thomakos N, Haidopoulos D, Lykka M, Koutsoukos K, Kostouros E, Terpos E, Chatziioannou A, Dimopoulos MA, Bamias A. Trachana SP, et al. PLoS One. 2016 Jun 3;11(6):e0156403. doi: 10.1371/journal.pone.0156403. eCollection 2016. PLoS One. 2016. PMID: 27258020 Free PMC article. - Extracellular vesicle-mediated phenotype switching in malignant and non-malignant colon cells.
Mulvey HE, Chang A, Adler J, Del Tatto M, Perez K, Quesenberry PJ, Chatterjee D. Mulvey HE, et al. BMC Cancer. 2015 Aug 1;15:571. doi: 10.1186/s12885-015-1568-3. BMC Cancer. 2015. PMID: 26231887 Free PMC article. - Transfer of extracellular vesicles during immune cell-cell interactions.
Gutiérrez-Vázquez C, Villarroya-Beltri C, Mittelbrunn M, Sánchez-Madrid F. Gutiérrez-Vázquez C, et al. Immunol Rev. 2013 Jan;251(1):125-42. doi: 10.1111/imr.12013. Immunol Rev. 2013. PMID: 23278745 Free PMC article. Review.
References
- André F, Schartz NE, Chaput N, Flament C, Raposo G, Amogorena S, Angevin E, Zitvogel L (2002a) Tumour-derived exosomes: a new source of tumour rejection antigens. Vaccine 20: A28–A31 - PubMed
- André F, Schartz NEC, Movassagh M, Flament C, Pautier P, Morice P, Pomel C, Lhomme C, Escudier B, LeChevalier T, Tursz T, Amigorena S, Raposo G, Angevin E, Zitvogel L (2002b) Malignant effusions and immunogenic tumour-derived exosomes. Lancet 360: 295–305 - PubMed
- Brown R, Clugston C, Burns P, Edlin A, Vasey P, Vojtesek B, Kaye SB (1993) Increased accumulation of p53 protein in cisplatin-resistant ovarian cell lines. Int J Cancer 55: 678–684 - PubMed
- Chaput N, Schartz NE, André F, Zitvogel L (2003) Exosomes for immunotherapy of cancer. Adv Exp Med Biol 532: 215–221 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials