Reversible silencing of CFTR chloride channels by glutathionylation - PubMed (original) (raw)
Reversible silencing of CFTR chloride channels by glutathionylation
Wei Wang et al. J Gen Physiol. 2005 Feb.
Abstract
The cystic fibrosis transmembrane conductance regulator (CFTR) is a phosphorylation- and ATP-dependent chloride channel that modulates salt and water transport across lung and gut epithelia. The relationship between CFTR and oxidized forms of glutathione is of potential interest because reactive glutathione species are produced in inflamed epithelia where they may be modulators or substrates of CFTR. Here we show that CFTR channel activity in excised membrane patches is markedly inhibited by several oxidized forms of glutathione (i.e., GSSG, GSNO, and glutathione treated with diamide, a strong thiol oxidizer). Three lines of evidence indicate that the likely mechanism for this inhibitory effect is glutathionylation of a CFTR cysteine (i.e., formation of a mixed disulfide with glutathione): (a) channels could be protected from inhibition by pretreating the patch with NEM (a thiol alkylating agent) or by lowering the bath pH; (b) inhibited channels could be rescued by reducing agents (e.g., DTT) or by purified glutaredoxins (Grxs; thiol disulfide oxidoreductases) including a mutant Grx that specifically reduces mixed disulfides between glutathione and cysteines within proteins; and (c) reversible glutathionylation of CFTR polypeptides in microsomes could be detected biochemically under the same conditions. At the single channel level, the primary effect of reactive glutathione species was to markedly inhibit the opening rates of individual CFTR channels. CFTR channel inhibition was not obviously dependent on phosphorylation state but was markedly slowed when channels were first "locked open" by a poorly hydrolyzable ATP analogue (AMP-PNP). Consistent with the latter finding, we show that the major site of inhibition is cys-1344, a poorly conserved cysteine that lies proximal to the signature sequence in the second nucleotide binding domain (NBD2) of human CFTR. This region is predicted to participate in ATP-dependent channel opening and to be occluded in the nucleotide-bound state of the channel based on structural comparisons to related ATP binding cassette transporters. Our results demonstrate that human CFTR channels are reversibly inhibited by reactive glutathione species, and support an important role of the region proximal to the NBD2 signature sequence in ATP-dependent channel opening.
Figures
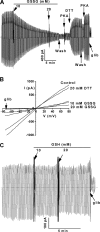
Figure 1.
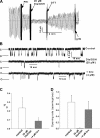
Reversible inhibition of CFTR channel activity by GSSG. Macroscopic currents were recorded for membrane patches excised from BHK-CFTR cells as described in
materials and methods
. (A) GSSG was added at the indicated final bath concentrations. The bath chamber was washed free of PKA and GSSG and then PKA (110 U/ml each addition), and 20 mM DTT was added as indicated. Glibenclamide (300 μM), a voltage-dependent blocker of CFTR current, was added at the end of the experiment. (B) Corresponding I–V curves showing voltage-independent inhibition of CFTR current by GSSG. (C) Modest effect of GSH on CFTR current. Dotted lines indicate zero current levels.
Figure 2.
CFTR inhibition by GSNO and diamide/GSH. (A) GSNO inhibition of CFTR currents. PKA inhibitory peptide (1.4 μg/ml PKI) was added at the first arrow. (B) Reversible inhibition of CFTR current by equimolar diamide/GSH. (C) CFTR channels are protected from diamide/GSH inhibition by pretreating the patch with NEM. NEM by itself stimulated the current as previously reported for CFTR (Cotton and Welsh, 1997). NEM, PKA, and PKI (but not MgATP) were washed out from the bath where indicated. (D) Mean data showing the inhibitory effects of GSNO (200 μM), GSSG (20 mM), and diamide/GSH (20 μM) on CFTR current and the rescue by subsequent addition of 20 mM DTT. Also shown is the protective effect of NEM pretreatment (0.1 mM) on the inhibition by diamide/GSH (20 μM). The results shown in Fig. 1 D were obtained following PKA inhibition with PKI (1.4 μg/ml). All data are normalized to the current recorded before the addition of the glutathione species. The number of experiments is shown above each bar.
Figure 3.
pH dependence of the inhibition of CFTR current by diamide/GSH. Macroscopic currents were recorded for membrane patches excised from BHK-CFTR cells at pH 7.3 (A), pH 6.3 (B), and pH 8.3 (C). Diamide and GSH were premixed at pH 7.3 and then added to the bath where indicated. Results are representative of four experiments. CFTR currents per se were only modestly affected by changing the pH over this range; e.g., changing the bath pH from 7.3 to 8.3 resulted in a 10–20% inhibition of macroscopic current (unpublished data).
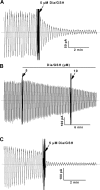
Figure 4.
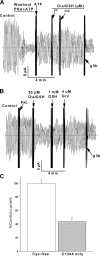
Glutaredoxin rescues CFTR channels from inhibition by reactive glutathione species. (A) Purified E. coli Grx1 (4 and 8 μM) reversed the inhibition of CFTR activity induced by diamide/GSH in an excised BHK-CFTR patch. (B) Purified E. coli Trx was unable to rescue CFTR activity following the addition of diamide/GSH. (C) CFTR activity also was rescued by a mutant Grx that is a specific mixed disulfide reductase (Grx3 C14S/C65Y). (D) Mean data showing the inhibitory effects of the indicated glutathione species (20 μM diamide/GSH; 200 μM GSNO; 20 mM GSSG) and the rescue effects of E. coli Grx1 (4 μM Grx1 plus 1 mM GSH) and GSH alone at high dose (20 mM). CFTR channels were preactivated with PKA and MgATP as described in
materials and methods
, and all additions were made after inhibiting the bath PKA with excess PKI inhibitory peptide (1.4 μg/ml). Results are normalized to the CFTR currents recorded before addition of the reactive glutathione species. The number of experiments is shown above each bar. For each glutathione species, the rescue by Grx or high GSH was statistically significant (P < 0.05) when compared with the current before Grx or GSH addition.
Figure 5.
Reversible glutathionylation of CFTR in isolated membrane vesicles. Microsomal membranes were prepared from BHK-CFTR cells as described in
materials and methods
. Microsomes (200 mg total protein) were incubated in PBS in the absence (lane 1) or presence of 125 μM biotin GSH and/or 100 μM diamide for 10 min at 21–23°C (lanes 2–7). Where indicated, 4 μM E. coli Grx1 (plus 1 mM GSH) (lane 5), 20 mM GSH (lane 6), or 20 mM DTT (lane 7) was added for an additional 15 min at 21–23°C. Microsomes were then solubilized, and biotin-containing proteins were isolated by pull-down with streptavidin-agarose and probed for CFTR by immunoblotting as described in
materials and methods
. Total CFTR protein was assayed by immunoprecipitating CFTR from 3% of the total crude membrane lysate and blotting the immunoprecipitate with the same CFTR monoclonal antibody. This experiment was repeated four times with similar results.
Figure 6.
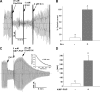
Glutathionylated CFTR channels exhibit markedly reduced open rates. (A) Macroscopic current trace showing inhibition of CFTR current by diamide/GSH in membrane patch excised from Calu-3 epithelial cell. PKI (1.4 μg/ml) was added at the arrow. (B) Single channel record obtained from Calu-3 patch showing marked inhibition of channel opening by diamide/GSH (20 μM) and partial recovery by E. coli Grx1 (4 μM Grx1 plus 1 mM GSH). Holding potential was −80 mV. (C) Mean data showing inhibitory effects of diamide/GSH (20 μM) and rescue effects of Grx1 (+1 mM GSH) on single channel open probability (Po) and channel opening rate (n = 3 patches). The results shown in B and C were obtained without PKI addition. Nearly identical results were obtained for two additional patches that were treated with diamide/GSH after PKI addition (unpublished data).
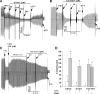
Figure 7.
CFTR channels that lack cys-1344 (C1344A-CFTR) are largely resistant to inhibition by reactive glutathione species. (A) Schematic view of CFTR depicting the 18 cysteines within the polypeptide and the specific location of cys-1344 near the ABC signature motif in NBD2. Bottom, sequences surrounding the NBD2 signature sequences in CFTR from human (h), mouse (m), and shark(s). (B) Mean data showing the inhibitory effects of diamide/GSH (20 μM) on the indicated alanine-substituted mutants and WT CFTR expressed in HEK-293T cells. Quad mutant is C128/225/343/866. Diamide/GSH was added after inhibiting the bath PKA with PKI. Data are normalized to currents measured just before the addition of diamide/GSH. (C) Representative current trace showing resistance of C1344A-CFTR to inhibition by diamide/GSH. (D) Mean data comparing the sensitivities of WT CFTR and C1344A-CFTR to inhibition by diamide/GSH (20 μM), GSNO (200 μM), and GSSG (20 mM).
Figure 8.
Cys-1344 is sufficient for reactive glutathione species to inhibit CFTR currents. Cys-free CFTR and cys-1344-only CFTR were expressed in HEK-293T cells as described in the text. (A) Representative current trace showing lack of inhibition of cys-free CFTR by diamide/GSH. To confirm that the observed currents were CFTR-mediated, PKA and MgATP were washed out of the bath and 1.5 mM MgATP was readded where indicated. (B) Representative current trace showing inhibition of the current mediated by the cys-1344-only construct by diamide/GSH. The currents mediated by both constructs were blocked by glibenclamide in a voltage-dependent manner, although they were inhibited by PKI to a smaller degree than that observed for WT channels (possibly because of the altered conformations of these ER processing mutants). (C) Mean data showing percent current remaining after the addition of 20 μM diamide/GSH (cys-free, n = 7; cys-1344-only, n = 8).
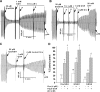
Figure 9.
An R domain deletion mutant is highly sensitive to diamide/GSH inhibition, whereas channels that are locked open by AMP-PNP are protected. (A) Inhibitory effect of 20 μM diamide/GSH on macroscopic current mediated by ΔR-S660A-CFTR in patch excised from transfected HEK-293T cell. (B) Mean data for ΔR-S660A-CFTR showing inhibition by diamide/GSH (20 μM) and recovery by E. coli Grx1 (4 μM) plus 1 mM GSH. Currents are normalized to control currents before diamide/GSH. Currents were activated with 1.5 mM MgATP in the absence of PKA for the experiments summarized in A and B. (C) Slow inhibition of CFTR currents by diamide/GSH following addition of 2 mM AMP-PNP to BHK-CFTR patch. Inset, exponential fit of current trace following addition of diamide/GSH to this patch. (D) Mean data comparing the exponential time constants for current inhibition by 20 μM diamide/GSH in the presence and absence of 2 mM AMP-PNP. Note that the time constants in the absence of AMP-PNP are overestimates due to the very rapid inhibition observed under the control condition.
Figure 10.
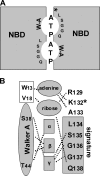
Schematic views of the regions proximal to the signature sequences in related ABC transporters. (A) Schematic view of ATP binding pockets sandwiched between NBD dimers based on structures of Rad50 (Hopfner et al., 2000) and MalK (Chen et al., 2003). (B) ATP binding pocket in the MalK dimer of the bacterial maltose transporter (adapted from Chen et al., 2003). Asterisks denote the positions corresponding to cys-1344 in CFTR NBD2.
Similar articles
- An electrostatic interaction at the tetrahelix bundle promotes phosphorylation-dependent cystic fibrosis transmembrane conductance regulator (CFTR) channel opening.
Wang W, Roessler BC, Kirk KL. Wang W, et al. J Biol Chem. 2014 Oct 31;289(44):30364-30378. doi: 10.1074/jbc.M114.595710. Epub 2014 Sep 4. J Biol Chem. 2014. PMID: 25190805 Free PMC article. - Prolonged nonhydrolytic interaction of nucleotide with CFTR's NH2-terminal nucleotide binding domain and its role in channel gating.
Basso C, Vergani P, Nairn AC, Gadsby DC. Basso C, et al. J Gen Physiol. 2003 Sep;122(3):333-48. doi: 10.1085/jgp.200308798. J Gen Physiol. 2003. PMID: 12939393 Free PMC article. - Regulation of the CFTR chloride channel from humans and sharks.
Hanrahan JW, Mathews CJ, Grygorczyk R, Tabcharani JA, Grzelczak Z, Chang XB, Riordan JR. Hanrahan JW, et al. J Exp Zool. 1996 Jul 1;275(4):283-91. doi: 10.1002/(SICI)1097-010X(19960701)275:4<283::AID-JEZ6>3.0.CO;2-L. J Exp Zool. 1996. PMID: 8759925 Review. - ATP hydrolysis cycles and the gating of CFTR Cl- channels.
Gadsby DC, Dousmanis AG, Nairn AC. Gadsby DC, et al. Acta Physiol Scand Suppl. 1998 Aug;643:247-56. Acta Physiol Scand Suppl. 1998. PMID: 9789567 Review.
Cited by
- Effect of S-nitrosoglutathione on renal mitochondrial function: a new mechanism for reversible regulation of manganese superoxide dismutase activity?
Patil NK, Saba H, MacMillan-Crow LA. Patil NK, et al. Free Radic Biol Med. 2013 Mar;56:54-63. doi: 10.1016/j.freeradbiomed.2012.12.001. Epub 2012 Dec 12. Free Radic Biol Med. 2013. PMID: 23246566 Free PMC article. - Regulation by reversible S-glutathionylation: molecular targets implicated in inflammatory diseases.
Shelton MD, Mieyal JJ. Shelton MD, et al. Mol Cells. 2008 May 31;25(3):332-46. Epub 2008 May 16. Mol Cells. 2008. PMID: 18483468 Free PMC article. Review. - Mechanisms of altered redox regulation in neurodegenerative diseases--focus on S--glutathionylation.
Sabens Liedhegner EA, Gao XH, Mieyal JJ. Sabens Liedhegner EA, et al. Antioxid Redox Signal. 2012 Mar 15;16(6):543-66. doi: 10.1089/ars.2011.4119. Epub 2012 Jan 6. Antioxid Redox Signal. 2012. PMID: 22066468 Free PMC article. Review. - S-CMC-Lys-dependent stimulation of electrogenic glutathione secretion by human respiratory epithelium.
Guizzardi F, Rodighiero S, Binelli A, Saino S, Bononi E, Dossena S, Garavaglia ML, Bazzini C, Bottà G, Conese M, Daffonchio L, Novellini R, Paulmichl M, Meyer G. Guizzardi F, et al. J Mol Med (Berl). 2006 Jan;84(1):97-107. doi: 10.1007/s00109-005-0720-y. Epub 2005 Nov 11. J Mol Med (Berl). 2006. PMID: 16283140 - Secondhand smoke inhibits both Cl- and K+ conductances in normal human bronchial epithelial cells.
Savitski AN, Mesaros C, Blair IA, Cohen NA, Kreindler JL. Savitski AN, et al. Respir Res. 2009 Nov 27;10(1):120. doi: 10.1186/1465-9921-10-120. Respir Res. 2009. PMID: 19943936 Free PMC article.
References
- Aleksandrov, L., A. Mengos, X.-B. Chang, A. Aleksandrov, and J.R. Riordan. 2001. Differential interactions of nucleotides at the two nucleotide binding domains of the cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 276(16):12918–12923. - PubMed
- Aracena, P., G. Sanchez, P. Donoso, S.L. Hamilton, and C. Hidalgo. 2003. S-glutathionylation decreases Mg2+ inhibition and S-nitrosylation enhances Ca2+ activation of RyR1 channels. J. Biol. Chem. 278(44):42927–42935. - PubMed
- Barrett, W.C., J.P. DeGnore, S. König, H.M. Fales, Y.-F. Keng, Z.-Y. Zhang, M.B. Yim, and P.B. Chock. 1999. Regulation of PTP1B via glutathionylation of the active site cysteine 215. Biochemistry. 38:6699–6705. - PubMed
- Berger, A.L., M. Ikuma, J.F. Hunt, P.J. Thomas, and M.J. Welsh. 2002. Mutations that change the position of the putative γ-phosphate linker in the nucleotide binding domains of CFTR alter channel gating. J. Biol. Chem. 277(3):2125–2131. - PubMed
- Bushweller, J.H., F. Åslund, K. Wüthrich, and A. Holmgren. 1992. Structural and functional characterization of the mutant Escherichia coli glutaredoxin (C14S) and its mixed disulfide with glutathione. Biochemistry. 31:9288–9293. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 DK053090/DK/NIDDK NIH HHS/United States
- DK56796/DK/NIDDK NIH HHS/United States
- R56 DK056796/DK/NIDDK NIH HHS/United States
- R01 DK056796/DK/NIDDK NIH HHS/United States
- DK53090/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous