TIEG1 null mouse-derived osteoblasts are defective in mineralization and in support of osteoclast differentiation in vitro - PubMed (original) (raw)
doi: 10.1128/MCB.25.3.1191-1199.2005.
Genevieve Gorny, Steven A Johnsen, David G Monroe, Glenda L Evans, Daniel G Fraser, David J Rickard, Kay Rasmussen, Jan M A van Deursen, Russell T Turner, Merry Jo Oursler, Thomas C Spelsberg
Affiliations
- PMID: 15657444
- PMCID: PMC543998
- DOI: 10.1128/MCB.25.3.1191-1199.2005
TIEG1 null mouse-derived osteoblasts are defective in mineralization and in support of osteoclast differentiation in vitro
Malayannan Subramaniam et al. Mol Cell Biol. 2005 Feb.
Abstract
Transforming growth factor beta-inducible early gene 1 (TIEG1) is a member of the Kruppel-like transcription factor family. To understand the physiological role of TIEG1, we generated TIEG(-/-) (null) mice and found that the TIEG(-/-) mice had increased osteoblast numbers with no increased bone formation parameters. However, when calvarial osteoblasts (OBs) were isolated from neonatal TIEG(-/-) and TIEG(+/+) mice and cultured in vitro, the TIEG(-/-) cells displayed reduced expression of important OB differentiation markers. When the OBs were differentiated in vitro by treatment with bone morphogenic protein 2, the OBs from TIEG(+/+) calvaria displayed several mineralized nodules in culture, whereas those from TIEG(-/-) mice showed no nodules. To characterize the OBs' ability to support osteoclast differentiation, the OBs from TIEG(+/+) and TIEG(-/-) mice were cultured with marrow and spleen cells from TIEG(+/+) mice. Significantly fewer osteoclasts developed when TIEG(-/-) OBs were used to support osteoclast differentiation than when TIEG(+/+) OBs were used. Examination of gene expression in the TIEG(-/-) OBs revealed decreased RANKL and increased OPG expression compared to TIEG(+/+) OBs. The addition of RANKL to these cocultures only partially restored the ability of TIEG(-/-) OBs to support osteoclast differentiation, whereas M-CSF alone or combined with RANKL had no additional effect on osteoclast differentiation. We conclude from these data that TIEG1 expression in OBs is critical for both osteoblast-mediated mineralization and osteoblast support of osteoclast differentiation.
Figures
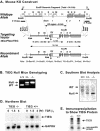
FIG. 1.
Mouse knockout construct and verification of TIEG1 null mutation. (A) Generation of the TIEG1 null allele (bottom panel) by homologous recombination between the targeting vector (middle panel) and wild-type allele (top panel). In the recombinant allele, the 1.8-kb Neor cassette replaces ca. 5.5 kb of genomic TIEG1 sequence, including exons 1 and 2. The locations of the 5′ and 3′ probes used in Southern analysis screening of transfected ES cell clones are indicated in the top panel. (B) PCR genotyping of mice generated from the mating of heterozygous TIEG1 mutant mice. The arrows indicate the products produced from the wild-type and mutant alleles. (C) Southern blot analysis was performed on genomic DNA from wild-type (TIEG+/+), heterozygous (TIEG+/−), and homozygous (TIEG−/−) TIEG1 mutant mice to verify genotypes. (D) In order to verify loss of TIEG1 expression, Northern analysis was performed as described in Materials and Methods. RNA was extracted from OBs grown in serum-free medium and treated with 2 ng of TGF-β/ml indicated times. (E) Immunoprecipitation analysis was performed from 35S-labeled cell lysates with TIEG-specific polyclonal antibody to demonstrate that TIEG−/− mice did not express TIEG1 protein.
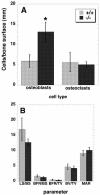
FIG. 2.
Histomorphometric analysis of mice. (A) Cell count for osteoblast and osteoclasts per surface area in cancellous bone (in millimeters). (B) Tissue and cellular measurements. The units differ for each data set and were as follows: LS/BS, the percentage of labeled cancellous bone surface per total measured cancellous bone surface; BFR/BS, the amount of new bone formed per week expressed as the percentage of bone surface; BFR/TV, the amount of new bone formed per week expressed as a percentage of total tissue volume; BV/TV, the percentage of cancellous bone volume per measured tissue volume; MAR, mineral apposition rate (>1 μm/week). Data are the means of 5 (+/+) or 6 (−/−) animals ± the SEM. ✽, P < 0.05.
FIG. 3.
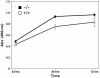
TIEG+/+ and TIEG−/− OBs were plated onto 96-well plates and the cells were grown for 24, 48, and 72 h at 37°C. The proliferation of these cells were measured by using cell titer 96 “the Aqueous One solution for cell proliferation assay” as described in Materials and Methods. An average of six replicates of each were graphed.
FIG. 4.
TIEG+/+ and TIEG−/− OBs were grown in differentiation medium with or without BMP2 for 18 days and stained with alizarin red to visualize the bone nodules.
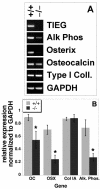
FIG. 5.
Total RNA was isolated from TIEG+/+ and TIEG−/− OBs grown in culture, and RT-PCR was performed for osteoblast-specific marker genes. (A) Agarose gel of the RT-PCR products as visualized with ethidium bromide. (B) RT-PCR was performed on five separate calvarial RNA isolates, and the results were scanned and quantitated by using NIH Image. The data are the means ± the SEM of these analyses normalized to GAPDH expression. ✽, P < 0.05 (comparing TIEG+/+ to TIEG−/− OBs).
FIG. 6.
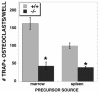
Knockout OBs are impaired in supporting osteoclast differentiation. Neonatal calvarium-derived osteoblasts from TIEG+/+ and TIEG−/− mice were cultured with either marrow or spleen osteoclast precursors from TIEG**+/+** mice in the presence of vitamin D and dexamethasone for 9 days as described in the text. The data are means ± the SEM of three replicate wells from one experiment. The experiment was performed four times, and these data are representative of the results. ✽, P < 0.05 (comparing TIEG+/+ to TIEG−/− calvarial cells).
FIG. 7.
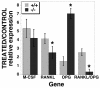
The RANKL/OPG ratio is lower in TIEG−/− cells than in TIEG+/+ cells. Calvarial cells were cultured with (treated) vitamin D and dexamethasone or without treatment (control), followed by RNA isolation as described in the text. Real-time PCR was performed to quantitate the M-CSF, RANKL, and OPG expression relative to tubulin, and the ratio of RANKL to OPG was determined. ✽, P < 0.05 (comparing TIEG+/+ to TIEG−/− calvarial cells).
FIG. 8.
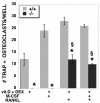
RANKL partly, but not completely, restores TIEG−/− calvarial cell ability to support osteoclast differentiation. TIEG+/+ and TIEG−/− calvarial cells were cultured with TIEG+/+ marrow in the presence of the hormones and/or growth factors indicated at the bottom of the figure. ✽, P < 0.05 (comparing TIEG+/+ to TIEG−/− calvarial OB cells); §, P < 0.05 [comparing vitamin D and dexamethasone alone to addition of the indicated growth factor(s)].
Similar articles
- Transforming growth factor beta affects osteoclast differentiation via direct and indirect actions.
Quinn JM, Itoh K, Udagawa N, Hausler K, Yasuda H, Shima N, Mizuno A, Higashio K, Takahashi N, Suda T, Martin TJ, Gillespie MT. Quinn JM, et al. J Bone Miner Res. 2001 Oct;16(10):1787-94. doi: 10.1359/jbmr.2001.16.10.1787. J Bone Miner Res. 2001. PMID: 11585342 - Roles of stromal cell RANKL, OPG, and M-CSF expression in biphasic TGF-beta regulation of osteoclast differentiation.
Karst M, Gorny G, Galvin RJ, Oursler MJ. Karst M, et al. J Cell Physiol. 2004 Jul;200(1):99-106. doi: 10.1002/jcp.20036. J Cell Physiol. 2004. PMID: 15137062 Free PMC article. - TGFbeta inducible early gene-1 directly binds to, and represses, the OPG promoter in osteoblasts.
Subramaniam M, Hawse JR, Bruinsma ES, Grygo SB, Cicek M, Oursler MJ, Spelsberg TC. Subramaniam M, et al. Biochem Biophys Res Commun. 2010 Jan 29;392(1):72-6. doi: 10.1016/j.bbrc.2009.12.171. Epub 2010 Jan 6. Biochem Biophys Res Commun. 2010. PMID: 20059964 Free PMC article. - Regulatory mechanisms of osteoblast and osteoclast differentiation.
Katagiri T, Takahashi N. Katagiri T, et al. Oral Dis. 2002 May;8(3):147-59. doi: 10.1034/j.1601-0825.2002.01829.x. Oral Dis. 2002. PMID: 12108759 Review. - RANK-Fc: a therapeutic antagonist for RANK-L in myeloma.
Sordillo EM, Pearse RN. Sordillo EM, et al. Cancer. 2003 Feb 1;97(3 Suppl):802-12. doi: 10.1002/cncr.11134. Cancer. 2003. PMID: 12548579 Review.
Cited by
- Transcription Factor KLF14 and Metabolic Syndrome.
Yang Q, Civelek M. Yang Q, et al. Front Cardiovasc Med. 2020 May 27;7:91. doi: 10.3389/fcvm.2020.00091. eCollection 2020. Front Cardiovasc Med. 2020. PMID: 32548128 Free PMC article. Review. - KLF10 Mediated Epigenetic Dysregulation of Epithelial CD40/CD154 Promotes Endometriosis.
Delaney AA, Khan Z, Zheng Y, Correa LF, Zanfagnin V, Shenoy CC, Schoolmeester JK, Saadalla AM, El-Nashar S, Famuyide AO, Subramaniam M, Hawse JR, Khazaie K, Daftary GS. Delaney AA, et al. Biol Reprod. 2016 Sep;95(3):62. doi: 10.1095/biolreprod.116.140764. Epub 2016 Aug 3. Biol Reprod. 2016. PMID: 27488034 Free PMC article. - TIEG1-null tenocytes display age-dependent differences in their gene expression, adhesion, spreading and proliferation properties.
Haddad O, Gumez L, Hawse JR, Subramaniam M, Spelsberg TC, Bensamoun SF. Haddad O, et al. Exp Cell Res. 2011 Jul 15;317(12):1726-35. doi: 10.1016/j.yexcr.2011.05.007. Epub 2011 May 18. Exp Cell Res. 2011. PMID: 21620830 Free PMC article. - Krüppel-like factor KLF10 regulates transforming growth factor receptor II expression and TGF-β signaling in CD8+ T lymphocytes.
Papadakis KA, Krempski J, Reiter J, Svingen P, Xiong Y, Sarmento OF, Huseby A, Johnson AJ, Lomberk GA, Urrutia RA, Faubion WA. Papadakis KA, et al. Am J Physiol Cell Physiol. 2015 Mar 1;308(5):C362-71. doi: 10.1152/ajpcell.00262.2014. Epub 2014 Dec 3. Am J Physiol Cell Physiol. 2015. PMID: 25472963 Free PMC article. - KLF transcription factors in bone diseases.
Wang H, Han J, Dmitrii G, Ning K, Zhang XA. Wang H, et al. J Cell Mol Med. 2024 Apr;28(8):e18278. doi: 10.1111/jcmm.18278. J Cell Mol Med. 2024. PMID: 38546623 Free PMC article. Review.
References
- Bieker, J. J. 2001. Krüppel-like factors: three fingers in many pies. J. Biol. Chem. 276:34355-34358. - PubMed
- Borton, A. J., J. P. Frederick, M. B. Datto, X. F. Wang, and R. S. Weinstein. 2001. The loss of Smad3 results in a lower rate of bone formation and osteopenia through dysregulation of osteoblast differentiation and apoptosis. J. Bone Miner. Res. 16:1754-1764. - PubMed
- Chalaeux, E., T. Lopez-Rovira, J. L. Rosa, Pons, G., L. M. Boxer, R. Bartrons, and F. Ventura. 1999. A zinc-finger transcription factor induced by TGFβ promotes apoptotic cell death in epithelial Mv1Lu cells. FEBS Lett. 457:478-482. - PubMed
- Cook, T., B. Gebelein, M. Belal, K. Mesa, R., and Urrutia. 1999. Three conserved transcriptional repressor domains are a defining feature of the TIEG1 subfamily of Sp1-like zinc finger proteins. J. Biol. Chem. 274:29500-29504. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous